Abstract
The hereditary periodic fevers are a group of Mendelian disorders characterized by seemingly unprovoked fever and localized inflammation. Recent data indicate that these illnesses represent inborn errors in the regulation of innate immunity. Pyrin, the protein mutated in familial Mediterranean fever, defines an N-terminal domain found in a large family of proteins involved in inflammation and apoptosis. Through this domain pyrin may play a role in the regulation of interleukin (IL)-1β, nuclear factor (NF)-κB, and leukocyte apoptosis. Cryopyrin/NALP3, another protein in this family, is mutated in three other hereditary febrile syndromes and participates in the inflammasome, a newly recognized macromolecular complex crucial to IL-1β activation. Somewhat unexpectedly, mutations in the 55 kDa receptor for tumor necrosis factor also give rise to a dominantly inherited periodic fever syndrome, rather than immunodeficiency, a finding that has stimulated important investigations into both pathogenesis and treatment. Finally, the discovery of the genetic basis of the hyperimmunoglobulinemia D with periodic fever syndrome suggests an as yet incompletely understood connection between the mevalonate pathway and the regulation of cytokine production. These insights extend our understanding of the regulation of innate immunity in man, while providing the conceptual basis for the rational design of targeted therapies, both for the hereditary periodic fevers themselves and other inflammatory disorders as well.
The hereditary periodic fever syndromes are a group of disorders characterized by recurrent episodes or, in some cases, fluctuating degrees of fever and localized inflammation,1,2 initially described as affecting primarily the serosal and synovial surfaces and the skin, but now recognized to include a somewhat broader distribution of affected tissues. The prototypic and probably most common hereditary periodic fever syndrome is familial Mediterranean fever (FMF). The hereditary periodic fevers now also include the tumor necrosis factor (TNF) receptor–associated periodic syndrome (TRAPS), the hyperimmunoglobulinemia D with periodic fever syndrome (HIDS), and three different clinical disorders all caused by mutations in CIAS1, which encodes a protein denoted cryopyrin or NALP3.
The hereditary periodic fevers differ from autoimmune diseases such as systemic lupus erythematosus and rheumatoid arthritis in that they lack high-titer autoantibodies or antigen-specific T-cells; they are termed autoinflammatory diseases (Table 1 ) to highlight this distinction.2 A number of other illnesses have subsequently been included under the autoinflammatory rubric, including Mendelian disorders such as Blau syndrome, as well as conditions with a more complex mode of inheritance, such as Behçet’s disease. Recent advances in the genetics and molecular biology of the hereditary periodic fever syndromes have defined important new gene families and pathways in the regulation of innate immunity, thus substantiating the distinction from autoimmune disorders, which more directly affect the adaptive immune system. Insights into the pathogenesis of the hereditary periodic fevers have also catalyzed dramatic advances in targeted biologic therapies for some of these conditions.
Familial Mediterranean Fever
FMF is a recessively-inherited disorder typically presenting in childhood or adolescence with 1- to 3-day episodes of fever often accompanied by severe abdominal pain, pleurisy, monoarticular arthritis, or an erythematous rash on the ankle or foot known as erysipeloid erythema (Table 2 ). During attacks there is usually neutrophilia and a brisk acute-phase response, and histologically there is a massive (sterile) influx of polymorphonuclear leukocytes into the affected anatomic compartment(s). Between attacks, patients feel well, although biochemical evidence for inflammation may persist. Among some FMF patients, systemic amyloidosis develops due to the deposition of a misfolded fragment of serum amyloid A (SAA), one of the acute-phase proteins. FMF is most common among individuals of Jewish, Armenian, Turkish, Arab, and Italian ancestry, but, with the availability of genetic testing, cases have been documented worldwide. Treatment with daily oral colchicine markedly reduces the frequency and severity of FMF attacks, and usually prevents the development of amyloidosis,1 and thus with early diagnosis the prognosis of FMF is good.
The gene for FMF, MEFV, is located on human chromosome 16p (Table 3 ) and was independently identified by two positional cloning consortia in 1997.3,4MEFV is comprised of 10 exons, spanning approximately 15 kb of genomic DNA. All four of the initial FMF-associated mutations in MEFV were in exon 10, and even now, with over 55 mutations having been identified (available at http://fmf.igh.cnrs.fr/infevers), exon 10 remains the major site of mutations, with a smaller cluster in exon 2. Nearly all of the known FMF-associated mutations encode conservative missense changes. This suggests that the disease phenotype may require some level of residual protein function and that the episodic nature of FMF may be due to as yet uncharacterized environmental perturbations of the variant protein. Carrier frequencies for FMF mutations of 1:3 to 1:5 have been observed in several Mediterranean and Middle Eastern populations,5–8 raising the possibility of heterozygote selection. Intragenic convergence of single nucleotide polymorphism haplotypes strongly suggests independent ancient ancestral origins for ethnically diverse modern-day carriers of at least four different common mutations.4,9 Although genetic testing may help confirm the diagnosis of FMF, particularly in countries in which the disease is uncommon, there are substantial numbers of patients with the clinical picture of FMF who have only one identifiable mutation, and even some patients with no identifiable mutations in MEFV, and thus clinical judgment remains important in establishing the diagnosis.1
Consistent with the biology of FMF, MEFV is expressed predominantly in granulocytes, monocytes, dendritic cells, and in fibroblasts derived from skin, peritoneum, and synovium.10–12 It encodes a full-length 781 aa protein denoted pyrin or marenostrin. In transfection experiments full-length pyrin is exclusively cytoplasmic, while endogenous pyrin is cytoplasmic in monocytes but is predominantly nuclear in granulocytes, dendritic cells, and synovial fibroblasts.12,13
Although sui generis at the time pyrin was discovered, the domain encoded by exon 1 is now recognized as the prototype for a 92 aa motif, the PYRIN domain, that is found in 20 human proteins involved in the regulation of inflammation and apoptosis.14–17 This motif has a six alpha-helix structure similar to that of death domains, death effector domains, and caspase-recruitment domains, a configuration known to mediate homotypic interactions. Through cognate PYRIN-PYRIN interactions with the bipartite adaptor protein ASC (apoptosis-associated speck-like protein with a caspase recruitment domain, which is comprised of an N-terminal PYRIN domain and a C-terminal caspase recruitment domain), pyrin acts as an upstream regulator of interleukin (IL)-1β activation.18,19 Pyrin appears to have both inhibitory and potentiating effects on IL-1β production depending on experimental conditions, and thus the role of wildtype human pyrin in the IL-1 pathway remains controversial. There is also evidence that pyrin plays a role in regulating nuclear factor (NF)-κB activation and apoptosis, at least in part through its interactions with ASC.18–22 Perhaps reflecting the relevance of these PYRIN domain–mediated interactions to the development of autoinflammatory disease, mutations in exon 1 are extremely rare in FMF.
In contrast, mutations in the C-terminal B30.2/rfp/SPRY domain of pyrin, encoded by exon 10, predominate in FMF. This motif is found in proteins with a variety of different functions, and is thought to mediate protein-protein interactions. Some authors have suggested the possibility that the B30.2/rfp/SPRY domain of pyrin might be an intracellular domain that binds pathogens, similar to the leucine-rich repeat domain of cryopyrin/NALP3 (vide infra).19,23 This hypothesis is based on (1) the large number of FMF-associated mutations in this domain; (2) the evidence for heterozygote selection for exon 10 mutations in Mediterranean populations; (3) data suggesting selection for specific B30.2/rfp/SPRY sequences in primate evolution;23 and (4) evidence that the B30.2/rfp/SPRY domain of another protein, TRIM5a, blocks infection with certain retroviruses, and has undergone positive selection in primate evolution similar to pyrin.24,25 Nevertheless, at the time of this writing there is no direct experimental evidence that the C-terminal domain of pyrin binds pathogen-derived molecules.
TNF Receptor–Associated Periodic Syndrome
TRAPS is a dominantly-inherited disorder caused by mutations in the 55 kDa TNF receptor (TNFRSF1A), which is encoded on human chromosome 12p.26 Several years before the discovery of these TNF receptor mutations, a dominantly inherited periodic fever syndrome that later proved to be TRAPS was described in a family of Irish and Scottish ancestry whose illness had been termed familial Hibernian fever. Subsequent studies have demonstrated a very broad population distribution of patients with periodic fever and TNF receptor mutations, and hence the more ethnically neutral TRAPS terminology is usually preferred. To be consistent with the original description of TRAPS, mutations in the TNFRSF1A gene in the appropriate clinical setting are required to make the diagnosis, although there remain a number of patients with TRAPS-like illnesses who do not have identifiable mutations.1
As is the case for FMF, TRAPS presents in childhood or adolescence, but in TRAPS the duration of attacks tends to be more variable, ranging from short episodes of one to two days to flares lasting weeks at a time and, in some cases, patients experience nearly continuous, fluctuating symptoms. In addition to fever, common clinical features of TRAPS include abdominal pain (due to sterile peritonitis), pleurisy, periorbital edema, a migratory erythematous skin rash with underlying myalgia, arthralgia or frank arthritis, and scrotal pain. Marked leukocytosis and elevation of acute-phase reactants is usually seen during attacks, and often persists into the intercritical period. Systemic amyloidosis may occur in up to 10% of patients, with an increased risk observed in patients with mutations involving cysteine residues.27 Although the attacks of TRAPS can often be debilitating, amyloidosis appears to be the major factor limiting longevity.1
The p55 TNF receptor protein is 455 aa in length, including a 29 aa leader, a 182 aa extracellular domain, a 21 aa transmembrane domain, and a 223 aa intracellular region that includes a death domain. The extracellular domain is, in turn, divided into four cysteine-rich subdomains, each of which contains three disulfide-bonded pairs of cysteines that constrain the three-dimensional folding of the protein. To date all of the over 45 known TRAPS mutations (http://fmf.igh.cnrs.fr/infevers) reside in the extracellular domain of the protein, and most are in the first two cysteine-rich subdomains, with about half being missense substitutions at cysteine residues that disrupt normal disulfide bonding. To date no patients have been identified with mutations in the transmembrane or intracellular domains, with null mutations, or with mutations in the p75 TNF receptor, which is encoded on chromosome 1p.
The pathophysiology of TRAPS is complex. Initial studies of a family with the C52F TNFRSF1A mutation indicated a defect in activation-induced ectodomain shedding of p55 (but not p75) receptors.26 Since receptor shedding is thought to play a homeostatic role both by limiting available cell surface TNF receptors and by creating a pool of potentially antagonistic soluble receptors, it was attractive to hypothesize that the “shedding defect” observed in TRAPS patients might explain their autoinflammatory phenotype. Subsequent studies indicate that, while plausible, this hypothesis cannot fully explain the TRAPS phenotype, since the shedding defect is seen in patients with some, but not all, p55 mutations.26–29 Moreover, recent studies indicate a number of other functional abnormalities in mutant p55 receptors, including altered intracellular trafficking,30 impaired TNF binding,30 and a defect in TNF-induced leukocyte apoptosis.31
Patients with TRAPS generally do not respond to treatment with colchicine, which is highly effective in FMF, and, although they are responsive to high-dose corticosteroids, side effects are often limiting. The recognition of the role of the TNF-pathway has led to the introduction of etanercept, the soluble p75 TNFR:Fc fusion protein, in the treatment of TRAPS. Etanercept is effective in reducing, although usually not totally eliminating, clinical and laboratory evidence of inflammation in TRAPS,32–34 a result that is consistent with a role for both TNF-dependent and -independent pathways in the pathogenesis of TRAPS.
Hyperimmunoglobulinemia D with Periodic Fever Syndrome
HIDS is a recessively inherited periodic fever syndrome first described in the Netherlands and still diagnosed most often in Northern Europeans.35 Attacks often begin during the first year of life, lasting 3–7 days, and are sometimes triggered by childhood immunizations. Concomitant findings may include headache, abdominal pain, prominent cervical lymphadenopathy, polyarthralgia or polyarticular arthritis, diffuse maculopapular rash, and aphthous ulcerations.36 As in FMF and TRAPS, HIDS attacks are accompanied by leukocytosis and elevated acute-phase reactants. Serum polyclonal IgD levels are also elevated in most HIDS patients, regardless of whether they are experiencing an attack, but do not correlate with disease severity, either over time for any given patient or when comparing patients. Increased serum IgD levels have also been observed in the minority of patients with FMF 37 and TRAPS,38,39 but the magnitude is usually much less in these latter two conditions. In contrast with FMF or TRAPS, systemic amyloidosis is extremely rare in HIDS, although it has been reported.40
In 1999 two groups, using complementing positional and functional approaches, identified HIDS-associated mutations in MVK, an eleven-exon gene on chromosome 12q that encodes the mevalonate kinase (MK) enzyme.41,42 MK catalyzes the conversion of mevalonic acid to 5-phospho-mevalonic acid in the synthesis of a number of important molecules, including cholesterol, vitamin D, bile acids, steroid hormones, and nonsterol isoprenoids. HIDS-associated mutations leave some residual enzymatic activity, while more profound mutations in MVK that totally ablate enzymatic activity cause mevalonic aciduria, a rare condition manifesting not only periodic fevers but also a number of other severe developmental abnormalities. Currently over 35 HIDS-associated mutations have been described (http://fmf.igh.cnrs.fr/infevers), and they are distributed throughout the 396 aa MK protein. The mutations associated with mevalonic aciduria cluster around the active sites of the enzyme.43,44 Recognition of the molecular genetic basis of HIDS permits the use of genetic testing and/or screening for urinary mevalonate (during attacks) to help establish the diagnosis. This is especially helpful in the minority of patients with borderline or normal serum IgD levels.
In vitro studies suggest that HIDS-mutant MK functions best at 30°C, with progressive decreases in function at 37°C and 39°C.45 This observation may account for the association of HIDS attacks with immunizations and infections, and may also explain the increased urinary mevalonate levels observed during HIDS attacks. It is unlikely that IgD plays a primary role in the pathogenesis of HIDS, given the poor correlation of IgD levels with disease severity, and the identification of occasional patients with the HIDS phenotype and MVK mutations but persistently normal IgD levels.42,46,47 Diminished cholesterol biosynthesis also appears an unlikely mechanism of disease, since HIDS patients generally have low-normal serum cholesterol levels, and more severe disorders of cholesterol synthesis do not exhibit a HIDS-like phenotype. Instead, current thinking on the pathogenesis of HIDS focuses either on the accumulation of mevalonic acid or the shortage of isoprenoids. Favoring the latter hypothesis are in vitro data demonstrating accentuated IL-1β secretion by leukocytes from HIDS patients that can be reversed by the addition of farnesol or geranyl-geraniol.48 Possibly, the small guanoside triphosphate-binding proteins, which undergo farnesylation or geranylation, may be the link between the mevalonate pathway and the innate immune system.47 There are currently no established therapies for HIDS, although TNF inhibition and statins are investigational.1
The Cryopyrinopathies
The cryopryinopathies are a spectrum of clinical disorders caused by mutations in CIAS1,49–52 a nine-exon gene on chromosome 1q encoding a protein that has variously been denoted cryopyrin, NALP3, PYPAF1, or CATERPILLER 1.1. Although a number of overlap syndromes have been described, three relatively distinct clinical disorders are recognized: familial cold autoinflammatory syndrome (FCAS), Muckle-Wells syndrome (MWS), and neonatal-onset multisystem inflammatory disease (NOMID, also known as chronic infantile neurologic cutaneous and arthropathy [CINCA] syndrome). An urticaria-like rash is common to all of the cryopyrinopathies and is characterized histologically by infiltrates of lymphocytes and neutrophils rather than mast cells, indicating that it is not true urticaria. FCAS is characterized by episodes of fever, urticaria-like rash, and polyarthralgia, usually precipitated by generalized exposure to the cold and lasting about 12 hours. In MWS, attacks are not usually precipitated by cold exposure but include the same urticaria-like rash as well as fever, malaise, limb pain, and, at times, abdominal pain, conjunctivitis, and arthralgia. Sensorineural hearing loss and/or systemic AA amyloidosis may also develop. Patients with NOMID/CINCA, the most severe of the cryopyrinopathies, often exhibit nearly continuous disease activity that fluctuates in severity. In addition to the fevers, arthralgia, hearing loss, and amyloidosis seen in MWS, patients with NOMID/CINCA often develop a deforming arthropathy and central nervous system involvement that can include chronic aseptic meningitis, intellectual impairment, and loss of vision. Eosinophilia or coagulopathy can also be seen in some patients with NOMID/CINCA.
The full-length cryopyrin/NALP3 protein is 920 aa in length, and consists of three major domains: an N-terminal PYRIN domain (aa 13–83) similar to the N-terminal domain of pyrin, a central NACHT domain (aa 217–513) that appears to be important both in intra- and inter-molecular interactions, and a C-terminal tandem array of 7 leucine rich repeats (LRR, aa 697–920). Nearly all of the more than 40 known mutations in cryopyrin (http://fmf.igh.cnrs.fr/infevers) are missense substitutions in the NACHT domain, encoded by exon 3 of CIAS1.
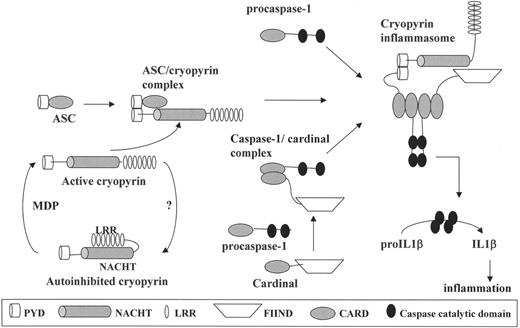
Cryopyrin/NALP3 has been shown to play an important role in the regulation of IL-1β activation through its participation in a macromolecular complex denoted the NALP3 inflammasome (Figure 1 ).53 In addition to cryopyrin/NALP3, this complex includes ASC, caspase-1 (which cleaves pro-IL-1β to produce biologically active IL-1β), and Cardinal, a caspase-recruitment domain-containing protein. The NALP3 inflammasome is a potent activator of IL-1β, and macrophages from patients with MWS spontaneously secrete active IL-1β. Through its LRR, cryopyrin/NALP3 binds muramyl dipeptide,54 a component of the bacterial cell wall, an event that increases inflammasome activity. Moreover, this process appears to be accentuated in the macrophages of patients with MWS.
The newly recognized role of cryopyrin/NALP3 in IL-1β activation has provided the conceptual basis for a series of studies establishing an important role for IL-1β inhibition in the cryopryinopathies. In one protocol, pretreatment with the IL-1β receptor antagonist anakinra effectively prevented clinical symptoms or acute-phase elevations in patients with FCAS exposed to cold temperatures.55 Anakinra also induced a complete remission of clinical symptoms and biochemical changes in MWS.56,57 Most recently, in a series of 18 patients with NOMID/CINCA, anakinra doses of 1–2 mg/kg/d resulted in a resolution of uveitis, rash, and fever and a significant decline in cerebrospinal fluid pressure.58 These results will not only dramatically improve the quality of life and prognosis of patients with a potentially devastating disease, but will provide powerful support for a model that places cryopyrin/NALP3 at a critical juncture in the regulation of IL-1β activation.
The systemic autoinflammatory diseases, a partial listing.
| Syndromes . | OMIM* . | Inheritance . | Genes or Risk Factors . |
|---|---|---|---|
| Other possible categories of autoinflammatory disease include metabolic disorders such as gout and pseudogout, storage diseases such as Gaucher’s disease and Hermansky-Pudkak syndrome, and fibrosing disorders such as idiopathic pulmonary fibrosis. | |||
| *Online Mendelian Inheritance in Man, an online catalog of genetic disorders available at http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=OMIM | |||
| Hereditary periodic fever syndromes | |||
| Familial Mediterranean fever (FMF) | 249100 | Autosomal recessive | MEFV |
| TNF receptor–associated periodic syndrome (TRAPS) | 142680 | Autosomal dominant | TNFRSF1A |
| Hyperimmunoglobulinemia D with periodic fever syndrome (HIDS) | 260920 | Autosomal recessive | MVK |
| Familial cold autoinflammatory syndrome (FCAS) | 120100 | Autosomal dominant | CIAS1/NALP3/PYPAF1 |
| Muckle-Wells syndrome (MWS) | 191100 | Autosomal dominant | CIAS1/NALP3/PYPAF1 |
| Neonatal-onset multisystem inflammatory disease (NOMID)/chronic infantile neurologic cutaneous and articular (CINCA) syndrome | 607115 | Sporadic, autosomal dominant | CIAS1/NALP3/PYPAF1 |
| Idiopathic febrile syndromes | |||
| Syndrome of periodic fever with aphthous stomatitis, pharyngitits, and cervical adenopathy (PFAPA) | —- | Not usually familial | — |
| Systemic-onset juvenile idiopathic arthritis (SOJIA) | 604302 | Complex | IL-6, MIF polymorphisms |
| Adult-onset Still’s disease | — | Not usually familial | — |
| Granulomatous disorders | |||
| Crohn’s disease | 266600 | Complex | NOD2/CARD15, ABCB1 (Ala893), MEFV (?) |
| Chronic granulomatous synovitis with uveitis and cranial neuropathy (Blau syndrome) | 186580 | Autosomal dominant | NOD2/CARD15 |
| Early onset sarcoidosis | 609464 | Sporadic, autosomal dominant | NOD2/CARD15 |
| Pyogenic disorders | |||
| Syndrome of pyogenic arthritis with pyoderma gangrenosum and acne (PAPA) | 604416 | Autosomal dominant | PSTPIP1 |
| Chronic recurrent multifocal osteomyelits (CRMO) | 259680 | Sporadic, autosomal recessive | LPIN2, when associated with congenital dyserythropoietic anemia (Majeed syndrome) |
| Synovitis, acne, pustulosis, hyerostosis, and osteitis syndrome (SAPHO) | — | Not usually familial | — |
| Hemophagocytic disorders | |||
| Primary hemophagocytic lymphohistiocytosis | 603553, 607624 | Autosomal recessive | PRF1, RAB27A |
| Macrophage activation syndrome (MAS) | — | Not usually familial | Pediatric rheumatic diseases |
| Complement disorders | |||
| Hereditary angioedema | 106100 | Autosomal dominant | C1NH |
| Vasculitic syndromes | |||
| Behçet’s disease | 109650 | Complex | HLAB51 |
| Syndromes . | OMIM* . | Inheritance . | Genes or Risk Factors . |
|---|---|---|---|
| Other possible categories of autoinflammatory disease include metabolic disorders such as gout and pseudogout, storage diseases such as Gaucher’s disease and Hermansky-Pudkak syndrome, and fibrosing disorders such as idiopathic pulmonary fibrosis. | |||
| *Online Mendelian Inheritance in Man, an online catalog of genetic disorders available at http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=OMIM | |||
| Hereditary periodic fever syndromes | |||
| Familial Mediterranean fever (FMF) | 249100 | Autosomal recessive | MEFV |
| TNF receptor–associated periodic syndrome (TRAPS) | 142680 | Autosomal dominant | TNFRSF1A |
| Hyperimmunoglobulinemia D with periodic fever syndrome (HIDS) | 260920 | Autosomal recessive | MVK |
| Familial cold autoinflammatory syndrome (FCAS) | 120100 | Autosomal dominant | CIAS1/NALP3/PYPAF1 |
| Muckle-Wells syndrome (MWS) | 191100 | Autosomal dominant | CIAS1/NALP3/PYPAF1 |
| Neonatal-onset multisystem inflammatory disease (NOMID)/chronic infantile neurologic cutaneous and articular (CINCA) syndrome | 607115 | Sporadic, autosomal dominant | CIAS1/NALP3/PYPAF1 |
| Idiopathic febrile syndromes | |||
| Syndrome of periodic fever with aphthous stomatitis, pharyngitits, and cervical adenopathy (PFAPA) | —- | Not usually familial | — |
| Systemic-onset juvenile idiopathic arthritis (SOJIA) | 604302 | Complex | IL-6, MIF polymorphisms |
| Adult-onset Still’s disease | — | Not usually familial | — |
| Granulomatous disorders | |||
| Crohn’s disease | 266600 | Complex | NOD2/CARD15, ABCB1 (Ala893), MEFV (?) |
| Chronic granulomatous synovitis with uveitis and cranial neuropathy (Blau syndrome) | 186580 | Autosomal dominant | NOD2/CARD15 |
| Early onset sarcoidosis | 609464 | Sporadic, autosomal dominant | NOD2/CARD15 |
| Pyogenic disorders | |||
| Syndrome of pyogenic arthritis with pyoderma gangrenosum and acne (PAPA) | 604416 | Autosomal dominant | PSTPIP1 |
| Chronic recurrent multifocal osteomyelits (CRMO) | 259680 | Sporadic, autosomal recessive | LPIN2, when associated with congenital dyserythropoietic anemia (Majeed syndrome) |
| Synovitis, acne, pustulosis, hyerostosis, and osteitis syndrome (SAPHO) | — | Not usually familial | — |
| Hemophagocytic disorders | |||
| Primary hemophagocytic lymphohistiocytosis | 603553, 607624 | Autosomal recessive | PRF1, RAB27A |
| Macrophage activation syndrome (MAS) | — | Not usually familial | Pediatric rheumatic diseases |
| Complement disorders | |||
| Hereditary angioedema | 106100 | Autosomal dominant | C1NH |
| Vasculitic syndromes | |||
| Behçet’s disease | 109650 | Complex | HLAB51 |
Clinical features of the hereditary periodic fever syndromes.

Abbreviations: FMF, familial Mediterranean fever; TRAPS, tumor necrosis factor receptor-associated periodic syndrome; HIDS, hyperimmunoglobulinemia D with periodic fever syndrome; FCAS, familial cold autoinflammatory syndrome; MWS, Muckle-Wells syndrome; NOMID/CINCA, neonatal-onset multisystem inflammatory disease/chronic infantile neurologic cutaneous and articular syndrome
Genetics and pathophysiology of the hereditary periodic fever syndromes.
| Disease . | Chromosome . | Gene . | Protein . | Proposed Pathophysiology . |
|---|---|---|---|---|
| Familial Mediterranean fever (FMF) | 16p13.3 | MEFV | Pyrin (also known as marenostrin) | Disorder in the regulation of interleukin (IL)-1β activation; possible abnormalities in nuclear factor (NF-κB) activation, leukocyte apoptosis, as well |
| TNF receptor–associated periodic syndrome (TRAPS) | 12p13 | TNFRSF1A | TNFRSF1A (TNFR1, p55, CD120a) | Impaired ectodomain cleavage of p55 receptor with stimulation; impaired trafficking of mutant receptors possibly causing constitutive NF-κB activation; impaired ligand-binding (resulting in preferential stimulation through p75?); impaired tumor necrosis factor (TNF)-stimulated leukocyte apoptosis |
| Hyperimmunoglobulinemia D with periodic fever syndrome (HIDS) | 12q24 | MVK | Mevalonate kinase (MK) | Accumulation of mevalonic acid, the substrate of the MK enzyme or deficiency in isoprenoids (products of the mevalonate pathway). Phenotype unlikely to be due to excessive IgD or cholesterol deficiency (another product of the mevalonate pathway). |
| The cryopyrinopathies (familial cold urticaria, Muckle-Wells, neonatal-onset multisystem inflammatory disease) | 1q44 | CIAS1 (also known as NALP3, PYPAF1, CATERPILLER 1.1) | Cryopyrin (or NALP3, PYPAF1, CATERPILLER 1.1) | Activating mutations in the cryopyrin/NALP3 protein, leading to increased activity of the inflammasome, and consequently excessive production of interleukin (IL)-1β; mutant cryopyrin shows increased sensitivity to activation by muramyl dipeptide. |
| Disease . | Chromosome . | Gene . | Protein . | Proposed Pathophysiology . |
|---|---|---|---|---|
| Familial Mediterranean fever (FMF) | 16p13.3 | MEFV | Pyrin (also known as marenostrin) | Disorder in the regulation of interleukin (IL)-1β activation; possible abnormalities in nuclear factor (NF-κB) activation, leukocyte apoptosis, as well |
| TNF receptor–associated periodic syndrome (TRAPS) | 12p13 | TNFRSF1A | TNFRSF1A (TNFR1, p55, CD120a) | Impaired ectodomain cleavage of p55 receptor with stimulation; impaired trafficking of mutant receptors possibly causing constitutive NF-κB activation; impaired ligand-binding (resulting in preferential stimulation through p75?); impaired tumor necrosis factor (TNF)-stimulated leukocyte apoptosis |
| Hyperimmunoglobulinemia D with periodic fever syndrome (HIDS) | 12q24 | MVK | Mevalonate kinase (MK) | Accumulation of mevalonic acid, the substrate of the MK enzyme or deficiency in isoprenoids (products of the mevalonate pathway). Phenotype unlikely to be due to excessive IgD or cholesterol deficiency (another product of the mevalonate pathway). |
| The cryopyrinopathies (familial cold urticaria, Muckle-Wells, neonatal-onset multisystem inflammatory disease) | 1q44 | CIAS1 (also known as NALP3, PYPAF1, CATERPILLER 1.1) | Cryopyrin (or NALP3, PYPAF1, CATERPILLER 1.1) | Activating mutations in the cryopyrin/NALP3 protein, leading to increased activity of the inflammasome, and consequently excessive production of interleukin (IL)-1β; mutant cryopyrin shows increased sensitivity to activation by muramyl dipeptide. |
Activation of interleukin (IL)-1β through the cryopyrin/NALP3 inflammasome. On the lower left, the major domain structure of cryopyrin is depicted. Interaction of cryopyrin with muramyl dipeptide (MDP) is thought to activate cryopyrin and allow it to interact with the other components of the inflammasome. In the upper left, activated cryopyrin binds ASC through cognate PYRIN-PYRIN domain interactions. ASC, in turn, binds caspase-1 through homotypic interactions of their caspase recruitment (CARD) domains (upper right). This complex then binds Cardinal, which has recruited a second caspase-1 molecule. The full assembly of this macromolecular complex, the inflammasome, induces proximity of the catalytic domains of the two caspase-1 molecules, leading to autocatalysis. The released catalytic domains are then available to activate pro-IL-1β to its biologically active form, which mediates fever and inflammation. Abbreviations: PYD, PYRIN domain; NACHT, the nucleotide-binding domain of cryopyrin; LRR, leucine-rich repeat; FIIND, a domain of Cardinal that interacts with cryopyrin; CARD, caspase-recruitment domain.
Activation of interleukin (IL)-1β through the cryopyrin/NALP3 inflammasome. On the lower left, the major domain structure of cryopyrin is depicted. Interaction of cryopyrin with muramyl dipeptide (MDP) is thought to activate cryopyrin and allow it to interact with the other components of the inflammasome. In the upper left, activated cryopyrin binds ASC through cognate PYRIN-PYRIN domain interactions. ASC, in turn, binds caspase-1 through homotypic interactions of their caspase recruitment (CARD) domains (upper right). This complex then binds Cardinal, which has recruited a second caspase-1 molecule. The full assembly of this macromolecular complex, the inflammasome, induces proximity of the catalytic domains of the two caspase-1 molecules, leading to autocatalysis. The released catalytic domains are then available to activate pro-IL-1β to its biologically active form, which mediates fever and inflammation. Abbreviations: PYD, PYRIN domain; NACHT, the nucleotide-binding domain of cryopyrin; LRR, leucine-rich repeat; FIIND, a domain of Cardinal that interacts with cryopyrin; CARD, caspase-recruitment domain.
From the Genetics and Genomics Branch, National Institute of Arthritis and Musculoskeletal and Skin Diseases, National Institutes of Health, Bethesda, MD