Abstract
The last three decades have seen tremendous improvements in survival of children diagnosed with cancer, with the 5-year survival rate approaching 80%. This improvement in survival has resulted in a growing population of childhood cancer survivors. Use of cancer therapy at an early age can produce complications that may not become apparent until years later. Approximately two thirds of the survivors of childhood cancer will experience at least one late effect, and about one third will experience a late effect that is severe or life threatening. Long-term complications in childhood cancer survivors, such as impairment in growth and development, neurocognitive dysfunction, cardiopulmonary compromise, endocrine dysfunction, renal impairment, gastrointestinal dysfunction, musculoskeletal sequelae, and subsequent malignancies, are related not only to the specific therapy employed, but may also be determined by individual host characteristics. We review the known late effects of treatment in survivors of childhood cancer in order to suggest reasonable starting points for evaluation of specific long-term problems in this unique but growing population. The Children’s Oncology Group (COG) has developed risk-based, exposure-related guidelines for follow-up care that are available at www.surivorshipguidelines.org.
Use of effective risk-based therapy for management of childhood cancer has resulted in tremendous improvements in overall survival over the last three decades, with the 5-year survival rate approaching 80%,1 resulting in a growing population of childhood cancer survivors. In 1997, there were an estimated 270,000 survivors of childhood cancer in the US, with 1 in 1000 individuals being a childhood cancer survivor.2 Demographics of the childhood cancer survivors in the US reveal that while a third of these survivors are still less than 20 years of age, 46% are between 20 and 40 years old and an additional 18% are over 40 years of age.
Cancer and its treatment during childhood can result in a variety of long-term sequelae, such as impairment in growth and development, neurocognitive dysfunction, cardiopulmonary compromise, endocrine dysfunction, renal impairment, gastrointestinal dysfunction, musculoskeletal sequelae, and subsequent malignancies. These sequelae are related not only to the specific therapy employed, but may also be determined by individual host characteristics. Furthermore, these long-term sequelae can have an adverse effect on the overall quality of life of the survivors. We will review some of the known late effects in survivors of childhood and the relationship between these effects and individual therapeutic exposures in order to suggest reasonable starting points for evaluation of specific long-term problems (Table 1 ).
Second Malignant Neoplasms
Follow-up studies of a large cohort of childhood cancer survivors have demonstrated a 3-fold increased risk of developing a second cancer when compared with the general population, and this risk continues to increase as the cohort ages.3 The incidence and the type of second malignancies differ with the primary diagnosis, type of therapy received, and presence of genetic conditions. The more commonly reported second malignant neoplasms in childhood cancer survivors are breast, thyroid and bone cancers, therapy-related myelodysplasia and acute myeloid leukemia. Secondary myelodysplasia and acute myeloid leukemia have been associated with certain chemotherapeutic agents, such as alkylating agents4 and topoisomerase II inhibitors.5 A dose-dependent relationship is noted with alkylating agents, which typically cause leukemias after latencies of 5 to 10 years. Cytogenetic abnormalities in the alkylating agent-associated secondary leukemias characteristically involve chromosomes 5 or 7. Topoisomerase II inhibitor-associated secondary leukemias classically have a shorter latency, no preceding dysplastic phase, and cytogenetic abnormalities involving chromosome 11q23. While the risk of solid tumors continues to climb with increasing follow-up, that of secondary leukemia plateaus after 10 years.6
Ionizing radiation is associated with several types of cancer, with the risk being highest when the exposure occurs at a younger age.3,4 The risk increases with the total dose of radiation7,8 and with increasing follow-up after radiation.9 Examples of radiation-associated tumors include breast and lung cancer, thyroid cancer, brain tumors, and osteosarcoma. Female patients treated with mantle radiation before the age of 21 years are at a significantly higher risk of developing radiation-related breast cancer in comparison with those treated in their adult years.6,10 The large majority of women are diagnosed with radiation-related breast cancer at a relatively young age, often before 40 years of age. An increased risk of developing thyroid cancer has been described after radiation therapy for several primary cancers, including Hodgkin disease, acute lymphoblastic leukemia, and brain tumors and after total body irradiation for hematopoietic cell transplantation. Increasing cumulative dose of radiation as well as exposures to radiation at a young age have been identified as risk factors.
Genetic predisposition may play a role in the development of second cancers, as evidenced by the increased risk of secondary sarcomas among patients with the genetic form of retinoblastoma, especially after increasing doses of radiation. Furthermore, members of families with Li-Fraumeni syndrome have been reported to be at increased risk of multiple subsequent cancers, with the highest risk observed among survivors of childhood cancer.11 It therefore appears that germline mutations in tumor suppressor genes, such as those occurring in the Li-Fraumeni syndrome, might interact with therapeutic exposures, resulting in an increased risk of second cancers.
Screening: Since subsequent malignancies remain a significant threat to the health of survivors treated for cancer during childhood, vigilant screening is important for those at risk. Risk for secondary acute myeloid leukemia usually manifests within 10 years following exposure. Monitoring should include annual complete blood count with differential and platelet count for 10 years post therapy. Most other subsequent malignancies are associated with radiation exposure. Screening recommendations include careful annual physical examination of the skin and soft tissues in the radiation field with radiographic or other cancer screening evaluations as indicated (such as dental examination if the mouth was in the radiation field). Specialized recommendations for females who received radiation with potential impact to the breast (i.e., mantle, mediastinal, whole lung) include monthly self-breast examination beginning at puberty, annual clinical breast examinations beginning at puberty until age 25 years, and then clinical breast examination every 6 months, with annual mammograms beginning 8 years after radiation or at age 25 (whichever occurs later). Screening of those at risk for early-onset colorectal cancer (i.e., radiation doses of 25 Gy or higher to the abdomen, pelvis, or spine) should include colonoscopy every 10 years beginning at age 35 years or 10 years following radiation (whichever occurs last). Patients with chronic hepatitis, most often resulting from blood product exposure prior to 1992, are at higher risk for developing hepatocellular carcinoma and should have serum alpha-fetoprotein monitoring on an annual basis; patients who develop cirrhosis should have an annual liver ultrasound.
Neurocognitive Sequelae
Neurocognitive sequelae of treatment for childhood cancer occur as a consequence of whole brain radiation, high-dose systemic methotrexate and/or cytarabine, or intrathecal methotrexate. Risk factors include increasing radiation dose, young age at the time of treatment, treatment with both cranial radiation and systemic or intrathecal chemotherapy, and female gender. Severe deficits are most frequently noted in children with brain tumors, especially those who were treated with radiation therapy, and in children who were younger than 5 years of age at the time of treatment. Neurocognitive deficits usually become evident within 1 to 2 years following radiation and are progressive in nature. Affected children are particularly prone to problems with receptive and expressive language, attention span, and visual and perceptual motor skills; irradiation-or chemotherapy-induced destruction in normal white matter partially explains intellectual and academic achievement deficits.12 They most often experience academic difficulties in the areas of reading, language, and mathematics. Children in the younger age groups treated with cranial radiation and those treated for brain tumors may experience significant drops in IQ scores.
Several studies have reported neuropsychological impairment in children with ALL exposed to central nervous system (CNS) therapy. 13–18 These reports indicate a significant decline in full-scale IQ in patients who received cranial irradiation, and this decline worsened with the length of time from diagnosis. Younger age at diagnosis was associated with lower full scale IQ in the radiated group.17,18 Several studies have indicated declines in cognitive function as a result of HCT performed in childhood or adolescence.19–25 Predictors of poor cognitive functioning have varied in different studies and have included younger age at HCT, lower socioeconomic status, pre-HCT cognitive functioning, conditioning regimen used, especially the use and dosage of TBI, particularly among children who were less than 3 years of age at exposure.19
Utilization of special education services has been shown to be significantly higher among childhood cancer survivors, in particular among leukemia and brain tumor survivors, when compared with age- and sex-matched sib-lings.26 Risk factors include younger age at diagnosis and exposure to cranial radiation with or without intrathecal methotrexate, with the risk increasing with the dose of radiation.
Screening: Neurocognitive complications in patients who received therapy that may potentially impact neuro-cognitive function should undergo a baseline neuropsychological evaluation, repeated as clinically indicated and at key transition points (e.g., when moving from grade school to middle/junior high school), as well as annual assessment of their vocational or educational progress.
Cardiovascular Function
The anthracyclines (e.g., doxorubicin, daunomycin and idarubicin) are well-known causes of cardiomyopathy.27 Chronic cardiotoxicity usually manifests itself as cardiomyopathy, pericarditis, and congestive heart failure. The incidence of cardiomyopathy is dose-dependent and may exceed 30% among patients who received a cumulative anthracycline dose in excess of 600 mg/m2. With a total dose of 500 to 600 mg/m2, the incidence is 11%, falling to less than 1% for cumulative doses less than 500 mg/m2.28 However, a lower cumulative dose of anthracyclines may place children at increased risk for cardiac compromise—a cumulative dose of greater than 250 mg/m2 (in association with radiation to the heart) was associated with a higher risk of clinical heart failure (cumulative incidence 20% at 25 years) compared with a cumulative dose lower than 250 mg/m2 (5%).29 The estimated risk of clinical heart failure increased with time and approached 19% after 25 years. Cardiomyopathy can occur many years after completion of therapy, and the onset may be spontaneous or coincide with exertion or pregnancy, especially during the third trimester. Chronic cardiac toxicity associated with radiation alone most commonly involves pericardial effusions or constrictive pericarditis, usually with radiation doses exceeding 40 Gy.30 Coronary artery disease has been reported following radiation to the mediastinum, with a cumulative risk of 21% at 20 years after radiation.31
Screening: Patients who received anthracycline chemotherapy need ongoing monitoring for late-onset cardiomyopathy, with frequency of evaluation based on total cumulative dose and age at the time of initial therapy. In addition to monitoring for cardiomyopathy, survivors who received radiation impacting the heart at doses > 30 Gy also need monitoring for potential early-onset atherosclerotic heart disease, valvular disease, and pericardial complications. Specific recommendations for monitoring based on age and therapeutic exposure, are delineated within the Children’s Oncology Group (COG) Long-term Follow-up guidelines (see below and www.survivorshipguidelines.org).
Pulmonary Function
Pulmonary radiation can lead to pulmonary fibrosis and pneumonitis. Clinically apparent pneumonitis with cough, fever, or dyspnea occurs in 5% to 15% of patients who received more than 30 Gy in standard fractions to more than 50% of the lung. Obstructive changes have also been reported after conventional radiation therapy. Following hematopoietic cell transplantation, both restrictive and obstructive lung disease including bronchiolitis obliterans are well described.32
Several chemotherapeutic agents are also responsible for pulmonary disease in long-term survivors. Interstitial pneumonitis and pulmonary fibrosis have been reported in children exposed to bleomycin,33 with the chronic lung toxicity being dose-dependent above a threshold cumulative dose of 400 units/m2 and exacerbated by concurrent or previous radiation therapy. As with bleomycin, carmustine (BCNU) and lomustine (CCNU) pulmonary toxicity is dose-related. Cumulative BCNU doses greater than 600 mg/m2 result in a 50% incidence of symptoms. Female patients are at a higher risk for this complication than their male counterparts.
Additional factors contributing to chronic pulmonary toxicity include superimposed infection, underlying pneumonopathy (e.g., asthma), cigarette smoking, respirator toxicity, chronic graft-versus-host disease, and the effects of chronic pulmonary involvement by tumor or reaction to tumor. Increased oxygen concentrations associated with general anesthesia or SCUBA diving also have been found to exacerbate pulmonary fibrosis.34
Screening: Monitoring for pulmonary dysfunction in childhood cancer survivors includes assessment of symptoms such as chronic cough or dyspnea on annual follow-up. Risks of smoking and exposure to second-hand smoke should be discussed with all patients. Pulmonary function tests (including DLCO and spirometry) and chest x-ray are recommended as a baseline upon entry into long-term follow-up for patients at risk, in patients with symptoms, or in those who require general anesthesia for any reason. Patients with risk factors for lung complications are discouraged from SCUBA diving.
Growth
Severe growth retardation, defined as a standing height below the fifth percentile, has been observed in as many as 30% to 35% of survivors of childhood brain tumors and in 10% to 15% of patients treated on certain antileukemia regimens.35,36 The effects of cranial irradiation are age-related, with children less than 8 years of age at the time of cranial irradiation at risk for adult height below the third percentile.36 Treatment with growth hormone prior to closure of epiphyses in patients with documented growth hormone deficiency usually results in near normalization of final height, unless the spinal axis has also been irradiated.
Screening: Monitoring of long-term survivors for growth problems relies on the use of standardized curves familiar to the pediatrician and available online (www.cdc.gov/growthcharts). Because single values for heights and weights are unreliable for children, frequent serial measurements should be obtained to establish each child’s pattern of growth. For children whose height crosses percentiles, are less than the third percentile, or whose growth velocity is less than 4 to 5 cm/year, endocrine consultation may be indicated.
Gonadal Function
Male gonadal function
All therapeutic modalities (radiation, surgery, or chemotherapy) cause both germ cell depletion and abnormalities of gonadal endocrine function among male cancer survivors. Radiation to the testes is known to result in germinal loss with decreases in testicular volume and sperm production, and increases in follicle-stimulating hormone (FSH). Effects are dose-dependent, following fractionated exposures of 0.1 to 6 Gy. Radiation therapy may also be toxic to Leydig cells, although at doses higher than those which are toxic to germ (Sertoli) cells. As summarized by Sklar,37 Leydig cell damage is dose-dependent and inversely related to age at treatment. Boys treated prepubertally or peripubertally with > 20 Gy for testicular leukemia, in addition to suffering germ cell depletion, are at high risk of delayed sexual maturation associated with decreased testosterone levels, despite increased luteinizing hormone (LH) levels. Adolescent and young adult male testes are relatively radioresistant, and fractionated doses greater than 30 Gy to the testes may induce Leydig cell failure in only about 50%.
Alkylating agents decrease spermatogenesis in a dose-dependent manner. Gonadal damage following cumulative doses of cyclophosphamide lower than 7.5 g/m2 (or 200 mg/kg, as used in hematopoietic cell transplantation) have been shown to be reversible in up to 70% of patients after therapy-free intervals of several years. In contrast to their prominent effects on germ cell epithelium, chemotherapy effects are less striking on slowly dividing Leydig cells and may be age-related. Following exposure to alkylating agents in prepubertal boys, normal pubertal progression and normal adult levels of testosterone are the rule; gynecomastia with low testosterone and increased LH have been reported in patients treated during adolescence, and compensated Leydig cell failure (increased LH with low normal testosterone levels or exaggerated FSH and LH responses to LH-releasing hormone) without gynecomastia is common in adults.38
Screening: Screening for problems related to male gonadal function in survivors include an annual age-appropriate history with specific attention to problems with libido, impotence, or fertility and examination for gynecomastia, Tanner staging of body hair, and assessment of penile and testicular size. Hormonal evaluation, including at least a single measurement of serum LH, FSH, and testosterone levels, is recommended as a baseline at age 14 years and in boys in whom puberty appears to be delayed. Males at risk of infertility may benefit from semen analysis; honest and sensitive discussions of fertility should be part of their follow-up visit. When abnormalities in testicular function are detected, close cooperation with an endocrinologist is essential in planning hormonal replacement therapy or in monitoring patients for spontaneous recovery. When no abnormalities are noted on history and physical examination but sexual maturity has not been completed, these studies should be repeated every 1 to 2 years. Conversely, in light of the potential for recovery of spermatogenesis and interpatient variations in gonadal toxicity, reminders about contraception should be given.
Female gonadal function
In contrast to the process in male survivors, germ cell failure and loss of ovarian endocrine function occur concomitantly in females. Radiation effects are both age- and dose-dependent. In women older than 40 years at the time of treatment, irreversible ovarian failure is an almost universal result of 4 to 7 Gy of conventionally fractionated radiation delivered to both ovaries. In contrast, prepubertal ovaries are relatively radioresistant, and despite higher doses (12–50 Gy), primary amenorrhea and delayed puberty eventually occurred in only 68% of patients treated at a mean age of 6.9 years.39 Secondary amenorrhea resulting from such modest doses appears to be reversible within several months to 4 years in 50% to 60% of patients.40 Total body irradiation (10 Gy single fraction) has been associated with primary amenorrhea and absent secondary sexual characteristics in most patients treated prior to puberty and followed for as long as 10 years.41 However, others have reported normal pubertal progression although with elevated FSH levels following total body irradiation during early childhood.42 As with standard radiation, increasing age at the time of total body irradiation has been found to predict ovarian failure.43 Premature menopause has also been reported in the setting of hematopoietic cell transplantation.41
Although chemotherapy-related gonadotoxicity is seen less frequently in females than in males, ovarian failure has been associated with chemotherapy, especially the alkylating agents, and the gonadotoxicity is dose- and age-dependent. Following myeloablative doses of alkylating agents, including busulfan and cyclophosphamide, permanent ovarian failure can be expected at all ages.44 For survivors who retain normal ovarian function after cancer therapy, there is an increased risk of premature menopause later in life.45 The risk factors associated with an early menopause include exposure to high doses of alkylating agents and abdomino-pelvic radiation.
Screening: The diagnostic evaluation of ovarian dysfunction relies on history (primary or secondary amenorrhea, menstrual irregularity, and pregnancies or difficulties becoming pregnant) and Tanner staging of breast and genital development. Serum gonadotropin (FSH, LH) and estradiol levels should be obtained as a baseline at age 13 years and as clinically indicated, in the absence of clinical evidence of puberty (menarche, development of secondary sexual characteristics), in order to assess the need for hormone therapy to induce puberty. In addition, because young women who have received gonadotoxic therapy and have progressed through puberty may experience early onset of menopause, they should also undergo assessment of gonadotropin and estradiol levels if there are clinical symptoms of estrogen deficiency (e.g., irregular menses, amenorrhea, hot flashes and vaginal dryness). Survivors with concerns regarding fertility are urged to seek consultation with reproductive endocrinologists.
Burden of Morbidity
Several large studies of childhood cancer survivors have described long-term sequelae associated with specific therapeutic exposures as well as late mortality experienced by the survivors that is in excess of the age- and sex-matched general population. However, little is known about the overall morbidity experienced by the survivor population. Some investigators have attempted to estimate the burden of morbidity by quantifying the chronic medical problems experienced by this population (Table 2 ).46–48 Chronic medical problems have been defined as health problems that cause physical, psychological or social difficulty and therefore justify ongoing medical intervention. These reports suggest that approximately two thirds of the survivors will experience at least one chronic medical problem and about one third will experience a late effect that is severe or life threatening, although psychosocial issues in survivors and family members may be more prevalent. In a recent study presented at the American Society of Clinical Oncology, Oeffinger et al described the prevalence and severity of chronic diseases experienced by a cohort of 10,397 adult survivors of childhood cancer and, utilizing a survivor comparison group of 3034 siblings, demonstrated that two-thirds of the survivors report at least one chronic health condition, one-third report a serious or life-threatening disease, and one-third report multiple health conditions.49 Individuals identified to be at highest risk included those who were treated for Hodgkin disease or brain tumors as well as those who had received chest radiation and anthracyclines. Overall, the survivors were at a fourfold higher risk of reporting a severe chronic health condition when compared with age- and sex-matched siblings.
These studies demonstrate quite conclusively that the implications of cure are not trivial and that, indeed, the burden of morbidity carried by childhood cancer survivors is quite substantial. Furthermore, these data support a critical need for continuing follow up of childhood cancer survivors into adult life and, more importantly, an imminent need to identify the resources to do so. There is also an urgent need for the survivors and their healthcare providers to be aware of the “at risk” populations in order to institute appropriate surveillance strategies.
Knowledge about Past Diagnosis and Treatment
An investigation of the childhood cancer survivors’ knowledge about past cancer diagnosis and treatment demonstrated that only 72% of the cancer survivors were able to accurately and precisely report their cancer diagnosis.50 Furthermore, although 94% of the cancer survivors reported past exposure to chemotherapy, only 52% of those having received doxorubicin could report exposure to the drug, and only 30% of those exposed to daunomycin could report the exposure accurately. Similarly, while exposure to radiation therapy was reported by 89%, only 70% of the childhood cancer survivors exposed to radiation could accurately describe the site of radiation. Most importantly, only 35% of the survivors understood that serious health problems could result from past treatment.
Health Care Utilization by Young Adult Survivors of Childhood Cancer
Healthcare utilization by a large cohort of long-term survivors of childhood cancer revealed that while 87% of the survivors reported general medical contact within the past two years, and 72% reported a general physical examination within the same time period, only 42% reported a cancer-related visit, and only 19% reported a visit to the cancer center.51 Furthermore, cancer-related visits declined with time since diagnosis, placing the burden on the general practitioner to provide ongoing care of these survivors. Factors associated with no contact with the healthcare system by these survivors included lack of health insurance, male sex, and lack of concern about future health.
Delivering Survivorship Care
Childhood cancer survivors, an especially high-risk population, seek and receive care from a wide variety of health care professionals, including oncologists, medical and pediatric specialists, surgeons, primary care physicians, gynecologists, nurses, psychologists and social workers.2 Providing appropriate health care for survivors of cancer is emerging as one of the major challenges in medicine. The challenge arises from the heterogeneity of this patient population treated with numerous therapeutic modalities in an era of rapidly advancing understanding of late effects. The Institute of Medicine has recognized the need for a systematic plan for lifelong surveillance that incorporates risks based on therapeutic exposures, genetic predisposition, health-related behaviors, and comorbid health conditions.2 Optimal healthcare delivery to this unique population requires the establishment of necessary infrastructure including several key components:52 (1) longitudinal care utilizing a comprehensive multidisciplinary team approach, (2) continuity, with a single healthcare provider coordinating needed services, and (3) an emphasis on the whole person, with sensitivity to the cancer experience and its impact on the entire family.
Although the number of childhood cancer survivors is ever increasing, health care professionals outside academic centers are unlikely to see more than a handful of survivors in their practice, and due to the heterogeneity of treatments received, there will likely be little similarity in their required follow-up care. Academic settings allow for establishment of a specialized multidisciplinary follow-up team to care for large numbers of survivors; however, the paucity of such centers and their limited geographic access make these specialized centers an option only for survivors who live nearby or who can afford time and expenses in order to travel to a distant center. Therefore, finding ways to educate survivors and their local healthcare providers regarding needed follow-up is a priority.
COG has developed risk-based, exposure-related guidelines (Long-Term Follow-Up Guidelines for Survivors of Childhood, Adolescent, and Young Adult Cancers)53 specifically designed to direct follow-up care for patients who have completed treatment for pediatric malignancies. These Guidelines represent a set of comprehensive screening recommendations that can be used to standardize and direct the follow-up care for this group of cancer survivors. Ongoing monitoring facilitates early identification of and intervention for treatment-related complications in order to increase quality of life for these patients. Specially tailored patient education materials, known as “Health Links” accompany the guidelines, offering detailed information on guideline-specific topics in order to enhance health promotion in this population with specialized healthcare needs. Examples of specific screening strategies outlined within the COG Long-Term Follow-Up Guidelines are summarized in Table 1 . The Guidelines and the Health Links can be downloaded from www.survivorshipguidelines.org.
Regardless of the setting for follow up, the first step in any evaluation is to have at hand an outline of the patient’s medical history and, most importantly, a treatment summary, with inclusion of the elements listed in Table 3 . Once completed, the treatment summary allows the survivors or their healthcare provider to interface with the COG Long-Term Follow-Up Guidelines to determine recommended follow-up care. Before the long-term survivor of childhood cancer graduates from the care of a pediatric oncologist, this treatment record and possible long-term problems should be reviewed with the family and, in the case of an adolescent or young adult, with the patient. Correspondence between pediatric oncologist and subsequent caretakers should address these same issues.
An interactive web-based version of a standardized summary form, designed to interface with an automated version of the COG Long-Term Follow-Up Guidelines in order to generate individualized follow-up recommendations, is currently under development and when completed will be available at www.survivorshipguidelines.org. This web-based resource should help to facilitate standardization and uniformity in the evaluation and follow-up care of the growing population of childhood cancer survivors with their unique healthcare needs.
Conclusions and Future Directions
The growing population of childhood cancer survivors carries a significant burden of morbidity, necessitating comprehensive follow-up of these survivors. This follow-up should ideally begin at the end of therapy, with a documented summarization of therapeutic exposures that dictates the use of recommendations within the long-term follow-up guidelines, thus ensuring standardization of care received by the survivors (Figure 1 ).
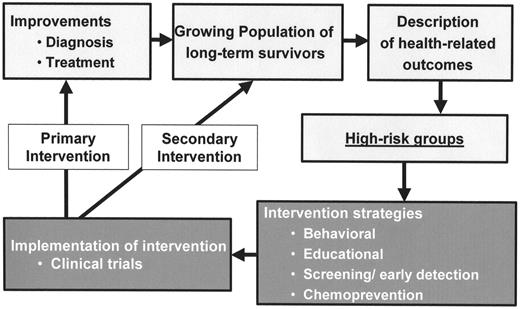
Improvement in childhood cancer diagnosis and treatment with the resultant growing population of survivors has also resulted in increasing emphasis on research focusing on adverse health-related outcomes and identification of high-risk groups (Figure 2 ). Attention now needs to focus on development of intervention strategies, such as behavior modification, educational intervention, screening for early detection of late effects, and chemoprevention. Execution of these intervention strategies in the setting of clinical trials would allow us to understand the impact of the specific interventions in early detection, with an overall reduction in morbidity and mortality and an ultimate improvement in the overall quality of life of childhood cancer survivors.
Supported in part by 5 U10 CA13539-26S2.