Abstract
With improvements in outcome, increased numbers of adult cancer patients survive free of their primary malignancy. Today, about 60% of adult patients diagnosed with cancer will survive 5 years after diagnosis. Therefore, immediate survival is no longer the sole concern. The aim of the cancer treatment now is to cure a patient’s underlying disease and, at the same time, to minimize the incidence of post-treatment complications and ensure the best possible long term quality of life. The long time span between initial therapy and late effects, the multiple factors influencing cancer-related health risk and the unknown effect of treatment on normal aging are common characteristics of late effects. While the treatment strategy for a cancer patient depends widely on the type and extension of the disease, considerations for a long-term survivor depend much more on the type of treatment applied, age of the patient, and the patient’s general health status as well as his or her familial and social integration. We discuss, based on the most recent knowledge, some typical examples of late effects in cancer survivors and the practical recommendations that could assist practitioner and patient decision about appropriate healthcare for specific clinical circumstances.
Cancer Survivorship and Identification of Patients at Risk
Cancer survivorship research seeks to identify diagnosis and treatment-related outcomes, provide a knowledge base regarding optimal follow-up care and surveillance of cancer survivors, and optimize health after cancer treatment.1 Long-term cancer survivors are defined as patients at least 5 years beyond the diagnosis of their primary disease. Late effects refer to long term changes in the health status of a cancer survivor that are often absent immediately after the cancer treatment but manifest later in a patient otherwise cured from the cancer. These late effects usually persist or worsen over time and may produce physical and psychological morbidity of variable intensity. Late effects may also have an impact on the patient’s relationship within the family and to society. However, not all patients will develop late complications. Many long-term survivors enjoy good health and report a good quality of life.2 Early detection of the patients with late complications may allow for early intervention without leading to an unnecessary expansion of the expenses in patients who are unlikely to develop late effects.
Late effects after cancer treatment include nonmalignant organ or tissue dysfunctions, secondary cancers, infections related to delayed or abnormal immune reconstitution, and changes in quality of life. The risk and the type of complication depend on the treatment received, the age of the patient at time of cancer treatment, the patient’s co-morbid conditions, and the time between the treatment and follow-up. The most significant treatment-related therapies affecting health status in long-term survivors are irradiation, chemotherapy, and allogeneic hemopoietic stem cell transplantation (HSCT). For an individual patient the knowledge of his risk profile for the development of late effects allows him to set up screening schedules and follow up recommendations as well as prevention and treatment guidelines. For the most part, recommendations for cancer survivors cannot be based on evidence derived from randomized or other controlled trials. Late effects are consequences and not study objectives. Therefore, recommendations are based on studies that have identified specific complications seen in long-term survivors.
Nonmalignant Late Effects
Nonmalignant late effects are heterogeneous in nature and intensity. The type and severity of these late effects depend on the type, duration and intensity of the treatment applied. Patients treated with irradiation are at particular risk for specific organ or tissue complications such as pulmonary late effects, liver complications, cardiac effects, ocular problems, late bone and joint complications, endocrine dysfunctions and fertility abnormalities. In fact any organ can be the target of a late complication in cancer survivors. In Table 1 the most important late effects occurring in adults, their risk factors, the monitoring parameters and recommendations are summarized. Many of the nonmalignant late effects in cancer survivors are discussed by Smita Bhatia in “Cancer Survivorship—Pediatric Issues” and by Leslie R. Schover in “Sexuality and Fertility after Cancer,” both in this volume. Therefore, only two characteristic examples, hypothyroidism and late liver complications, will be discussed in details.
Late thyroid dysfunction
The use of irradiation affecting the thyroid gland region is associated with increased risk of hypothyroidism from primary gland failure. The risk of thyroid dysfunction depends mainly on the cumulative irradiation dose and the time course after exposure. The cumulative incidence of overt hypothyroidism of 308 patients treated with loco-regional radiotherapy for non-thyroid head-and-neck cancer is 20% at 5 and 27% at 10 years.3 When hypothyroidism is defined as an increased serum thyroid-stimulating hormone (TSH), the cumulative incidence is even higher, 48% at 5 years and 67% at 8 years.4 After allogeneic HSCT, 7%–15% of the patients develop subclinical hypothyroidism.5 The frequency of hypothyroidism requiring therapy is highly variable depending on the type of pretransplant conditioning therapy applied. Single-dose total body irradiation (TBI) has higher incidence than fractionated-dose TBI and conditioning without irradiation. Onset of thyroid organ dysfunction varies. It starts usually around 5 years after irradiation, but may occur much later.
Recommendations: Patients treated with TBI, cervical, cranial, craniospinal, oropharyngeal, nasopharyngeal, mantel, mediastinal, or whole lung irradiation should be evaluated for thyroid function throughout their remaining life (Table 1 ). Treatment with L-thyroxin is indicated in all cases of frank hypothyroidism (elevated TSH with low free-T4 blood levels). Thyroid hormone levels should be measured after commencement of replacement therapy, and dosage should be tailored thereafter to the individual patient and adjusted as needed. Elderly patients should have an electrocardiogram prior to commencing treatment to exclude associated ischemic heart disease and/or arrhythmias.6
Late liver complications
Late liver complications may be difficult to assess in cancer survivors, because patients are often oligosymptomatic, may have several causes of liver dysfunction, and may have atypical patterns of viral serology. Hepatitis B (HBV) or hepatitis C (HCV) infections play a central role in late hepatopathy of cancer patients. They can occur due to transmission of the virus via transfusions of blood products and, in transplanted patients, via the graft. The hepatic injury often coincides with tapering of immunosuppressive treatment. The hepatitis may be asymptomatic, severe with progression to fulminant hepatitis, or evolve to chronic active hepatitis and cirrhosis. Before blood products were routinely screened the rate of posttransfusional hepatitis exceeded 20%. In patients receiving screened products, the rates of infection with HBV and HCV are 3.1% and 6%, respectively.7 Long-term studies of cancer survivors usually show a chronic liver disease with a mild course. However, in patients with a follow-up of more than 10 years, patients have more significant complications, including liver cirrhosis and hepatocellular carcinoma.8
Transplanted patients infected with HBV usually show a mild liver disease on long-term follow-up. Chronic hepatitis C is often asymptomatic with fluctuating transaminase levels over the years after HSCT. However, in HCV-positive long-term patients cirrhosis is a common late complication.9 Even asymptomatic patients with persistently normal alanine aminotransferase levels may eventually progress to cirrhosis.10 The cumulative incidence of cirrhosis in patients with hepatitis C after allogeneic HSCT is 11% and 24% at 15 and 20 years posttransplant, respectively.11 The risk of cirrhosis in transplanted recipients is significantly higher and median time to appear significantly shorter as compared to a control population. The increased risk of cirrhosis appears in long-term survivors over 10 years of follow-up. The role of possible risk factors for cirrhosis, such as iron overload, viral genotype or histological pattern, has not yet been elucidated.
Iron overload in cancer patients is essentially related to multiple transfusions and therefore is most commonly found in long-term survivors of acute leukemia or after HSCT (especially those for aplastic anemia or hemoglobinopathies). These patients may present hepatic dysfunction due to iron overload. Therapeutic phlebotomy can reduce iron overload and normalize ferritin and hepatic liver tests.12 After HSCT, up to 88% of long-term survivors have iron overload with high ferritin levels and high liver iron content.13 In addition to transfusion, prolonged dyserythropoiesis and increased iron absorption contribute to accumulation of iron. A clear correlation exists between iron overload and persistent hepatic dysfunction. However, the clinical consequences of iron overload and therapeutic iron depletion in transplant recipients have not been extensively evaluated. In heavily transfused patients, such as patients with thalassemia, iron accumulation may contribute to the development of liver fibrosis, cirrhosis and hepatocellular carcinoma.
Recommendations: In long-term survivors a liver function test should be monitored yearly. Patients with known HBV or HCV infection should be monitored for HbsAg and viral load by PCR. Liver biopsy and determination of alfa-feto protein should be considered in patients with chronic hepatitis B and C infection to determine extend of cirrhosis and detect hepatocellular carcinoma. The optimal treatment for hepatitis B and C after allogeneic HCT are areas of active research. In long-term survivors at risk for iron overload, serum ferritin and transferrin saturation should be monitored. Patients should be counseled to avoid iron intake and alcohol. In patients with documented liver iron content greater than 7 mg/g dry weight should be treated with phlebotomy and/or chelation therapy. The use of erythropoietin may facilitate phlebotomy in those with low hemoglobin levels.
Secondary Malignancies
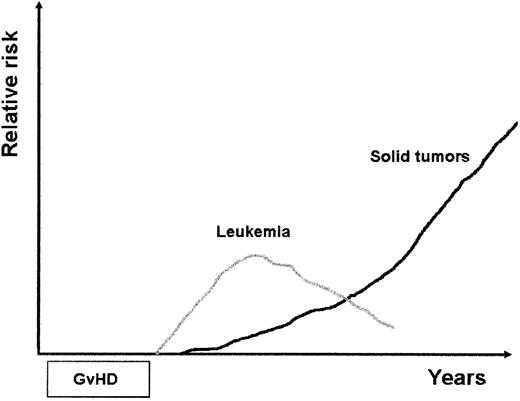
Adult cancer survivors have an increased risk of developing secondary malignant neoplasms. Secondary malignancies are a well-recognized complication in patients with Hodgkin disease or non-Hodgkin lymphoma treated with irradiation, chemotherapy or combined treatment modality.14,15 The increased risk of secondary cancers can be attributed to the mutagenic risk of irradiation and chemotherapy, the genetic predisposition of the patient to develop cancer and, in elderly patients, to age-related risk. Immunosuppressive treatment may play a role.16 Common cancers include leukemia and solid tumors. Secondary leukemias occur earlier after primary treatment than solid tumors, with a peak occurrence at 2 to 6 years, while solid cancers reveal with considerable delay presenting a continuous increasing risk even decades after remission (Figure 1 ).
Secondary leukemia
Secondary leukemias are mostly myelodysplastic syndromes or acute myeloid leukemia (MDS/AML). A majority of the patients with secondary leukemia have a clonal genetic marker, such as deletions in chromosomes 5 or 7 or a chromosomal anomaly on 11q23 or 12q22.17 These leukemias have a poor prognosis. The median survival time after diagnosis of secondary MDS/AML is 8 months, with a survival at 5 years of less than 10%. It is not clear whether the primary cancer diagnosis has an influence on the risk of developing secondary leukemia. The highest incidence of secondary leukemia has been reported in patients with lymphoma.18 Older age, a higher cumulative dose of alkylating agents, previous radiotherapy, or the use of TBI as conditioning regimen may have caused the higher incidence in lymphoma patients.19 The cumulative probability of therapy-related leukemia after autologous stem cell transplantation for lymphoma or Hodgkin disease ranges from 4% to 18%. The median time to development is 12–24 months after transplantation. Data of a randomized trial including 440 patients with indolent lymphoma demonstrate an increased risk of secondary hematological malignancies after myeloablative radiochemotherapy and autologous HSCT compared with conventional chemotherapy.20 After autologous HSCT 5 of 195 patients and after chemotherapy alone none of 235 patients developed a secondary hemopoietic neoplasm, with an estimated 5-year risk for secondary hematological malignancies of 3.8% and 0.0%, respectively.
Secondary leukemia after allogeneic HSCT is difficult to distinguish from relapse/evolution of the primary disease. After HSCT for nonmalignant indications the occurrence of a secondary leukemia is a rare event. There have been few reports on secondary leukemia in donor cells. The mechanism that would lead to leukemia in previously healthy donor cells is not clear.16,21
Secondary solid tumors
Secondary solid tumors after cancer treatment may arise in the breast, thyroid, gastrointestinal tract, lung, skin, urogenital tract, and brain. In patients treated previously with irradiation, the cancer is mainly found in the involved field of radiation. Solid tumors have been described in patients with lymphoma and Hodgkin disease after chemotherapy and irradiation treatment.22 Among 1319 patients with Hodgkin disease, 181 second malignancies and 18 third malignancies were observed. The risk increased with increasing radiation field size, with time after primary disease, and with combined modality therapy. The relative risk of second malignancy of 4.6 was significantly higher in patients receiving a combination therapy. The 5-year survival after development of a second malignancy was 38.1%, with the worst prognosis seen after acute leukemia and lung cancer. Female survivors of Hodgkin disease have a strongly elevated risk of secondary breast cancer.23 Risk factors associated with breast cancer development include age at irradiation, time since treatment and the radiation dose received.
The risk of secondary cancers following autologous transplantation has not been fully characterized.15 In a study on late mortality in survivors after autologous HSCT, 29% of long-term patients experienced a late death. Fifty-six percent of these late deaths were due to relapse of primary disease. Subsequent malignant neoplasms were the most frequent cause of non-relapse-related death, with a 12-fold increased risk compared to the general population.24 The incidence of secondary solid tumors is usually estimated lower than after allogeneic transplantation. Estimates of solid tumor incidence range from 1.0% to 4.9% at 10 years, and to 7.6% at 15 years.25 However, this incidence is likely to increase over time, as these curves show no evidence of plateauing. Risk factors include advanced age and autologous HSCT for lymphoma or Hodgkin disease. In a long-term follow-up study of a cohort of 605 patients treated with autologous HSCT for B-cell lymphoma, 42 solid tumors were observed with a cumulative incidence of solid tumors posttransplant of 10% at 10 years.15 The role of radiation exposure in inducing second malignancies, including conditioning with TBI or as involved-field irradiation, also remains controversial. The observed/expected ratio is particularly high for melanoma, breast and prostate cancers.15 In a single-center study of 800 patients treated with autologous HSCT, 16 solid tumors occurred at a median of 68 months following transplantation, with a 15-year cumulative incidence of 11%. The relative risk compared to the general population of developing a second malignancy was 1.98.
Patients undergoing allogeneic HSCT have an increased risk of new solid cancers. There is an increased risk over time after transplantation, with the greater risk among younger patients.25,26 The rate of new malignant disease is 2- to 4-fold higher than that in an age-matched control population.27,28 In a retrospective multicenter study of 19,229 patients who received allogeneic or syngeneic transplants the cumulative incidence rate for the development of a new solid cancer was 2.2% at 10 years and 6.7% at 15 years.27 The risk was significantly elevated for malignant melanoma and cancers of the buccal cavity, liver, brain, bone and connective tissue. Higher doses of TBI were associated with a higher risk of solid cancers. Chronic graft-versus-host disease (GVHD) and male sex were strongly linked with an excess risk of squamous-cell cancers of the buccal cavity and skin.29
Recommendations: Patients at risk for secondary malignancy should be encouraged to perform self-examination such as breast, oral cavity and skin examination, and to avoid high-risk behaviors such as smoking or excessive unprotected skin UV exposure. Life-long clinical assessment in yearly intervals should include symptom review, clinical examination and screening testing for secondary malignancies. In the event of unexpected changes in peripheral blood such as the appearance of macrocytosis, dysplastic changes of any cell line or cytopenia, bone marrow investigation should be performed.
Altered Immune Reconstitution
Cancer and cancer treatment may lead to immune deficiency. Innate immunity including recovery of granulocytes, monocytes, natural killer (NK) cells and dendritic cells occur rapidly following cancer treatment, whereas reconstitution of adaptive immunity with recovery a broad functional Band T-lymphocyte repertoire is much more delayed particularly after HSCT. The patients with this immune deficiency are at risk for infection. Late infections remain the major cause of non-relapse morbidity and mortality after allogeneic HSCT. The duration and severity of the immune deficiency after transplantation depends on the age of the patient, presence of certain viral infections (particularly those of the herpes virus family), preparative regimen, graft manipulation, graft source, and development of chronic GVHD. In cancer survivors not treated with allogeneic HSCT, infections occurring later than 2 years are rare events, with the exception of patients after splenectomy. Delayed immune reconstitution is related with the use of irradiation, T cell depletion, the use of purine analogue, or monoclonal antibodies such as rituximab or alemtuzumab.30
Alemtuzumab used for cancer treatment or as a conditioning regimen for HSCT is associated with high incidence of cytomegalovirus infection.31 Other types of opportunistic fungal or virus infections have been reported. Late infections with the use of rituximab are rare. We observed in a series of patients treated with chemotherapy and rituximab for advanced lymphoma a complete disappearance of B-lymphocytes with immeasurable immunoglobulin levels for more than 12 months (unpublished data). Patients with low CD4+ cell count are at risk for Pneumocystis carinii infection. A single course of cladribine, as applied in patients with hairy cell leukemia, decreases the CD4+ lymphocyte counts significantly. This decrease below the normal level is observed at median 40–50 months after treatment, but may persist over 5 years in some patients.32 The spleen plays a prominent role in host defence by filtering encapsulated bacteria such as Streptococcus pneumoniae once bloodstream invasion has occurred. Its absence results in an increased risk of serious sepsis. The overall incidence of septicemia remains low but death rates from overwhelming postsplenectomy infection are up to 600 times greater than in the general population.
Chronic GVHD is the leading cause of morbidity and mortality after allogeneic HSCT. The 5-year probability of late transplant-related mortality after HLA-identical HSCT is more than 20% in patients with GVHD and around 5% in patients without chronic GVHD. Despite improvement obtained in the prevention and treatment of GVHD the incidence and severity of chronic GVHD has not much changed over time. The use of an alternative donor, transplantation of older patients, donor lymphocyte infusion to consolidate or treat impending relapse and the increasing use of peripheral stem cells instead of marrow stem cells are the main reasons for this situation. Infection due to the GVHD-associated immune deficiency is the main cause of death in patients with chronic GVHD. Intensification of the conditioning regimen and T-cell depletion of the graft improves engraftment and prevents acute and chronic GVHD in mismatched transplants. T-cell depletion, however, leads to higher incidence of relapse of the underlying disease as well as to delayed immune reconstitution and occurrence of opportunistic infections.33,–36 The immunity of patients surviving 20 to 30 years after transplantation is normal or near normal.37 A low infection rate is reported in the very long-term survivors. This may be due to a complete immune reconstitution 20 years or longer after allogeneic HSCT. An alternative explanation is that patients with poor immune reconstitution have died earlier.
After allogeneic HSCT, myeloid cells and NK cells show the fastest reconstitution. Initial recovery of B cells occurs in the IgM level (2 to 6 months), followed by the IgG (3 to 12 months) and the IgA levels (6 to 36 months), recovery time depending on the conditioning, the graft characteristics and GVHD. The B-cell repopulation shows initially an oligoclonal pattern. It is not infrequent to find on electrophoretic analyses oligoclonal, biclonal or even monoclonal bands. Allogeneic recipients lose immune memory and therefore need to be revaccinated. The immune status has to be taken into consideration when considering vaccination of patients after HSCT. Antibody response is poor in patients with low CD4+ T-cell counts (< 100 × 106/L). Inactive, subunit or recombinant vaccines may simply be ineffective in transplant recipients, whereas live attenuated vaccines may be dangerous in immunocompromised patients.38
T-cell reconstitution occurs via two predominant pathways: a thymic-dependent pathway that recapitulates ontogeny and a thymic-independent pathway that involves expansion of mature T cells that survive the preparative regimen (recipient T-lymphocytes) or are contained within the graft (donor lymphocytes). The early T-cell expansion is mainly due to the thymic-independent pathway. Since GVH reaction is directly toxic to thymic microenvironment, GVHD contributes substantially to delayed immune reconstitution. However, the use of T-cell depletion to prevent GVHD also adversely effects immune reconstitution by limiting the efficiency of homeostatic peripheral expansion.
Recommendations: In daily practice, lymphocyte sub-population and particularly T helper lymphocyte (CD4+) counts are good markers of immune reconstitution. Reconstitution of B cells should be monitored by measurement of serum immunoglobulin levels. Patients who are immunocompromised should be educated regarding warning symptoms of infection and advised to seek early medical attention for infectious signs or symptoms. Transplanted patients with chronic GVHD should have antibiotic prophylaxis targeting encapsulated organisms given for as long as immunosuppressive therapy is administered. Surveillance with cytomegalovirus (CMV) antigen testing or PCR should be continued for allogeneic HSCT patients with chronic immunosuppression or chronic GVHD. All recipients should receive P carinii pneumonia (PCP) prophylaxis for 6 months or as long as immunosuppressive therapy is given for treatment/prevention of chronic GVHD. Nontrans-planted patients with low CD4+ counts should receive PCP prophylaxis as long as the CD4+ cells are below 200 × 106/L. Monitoring for the emergence of opportunistic infections, particularly for CMV infection, and use of appropriate prophylaxis is mandatory when using alemtuzumab clinically.39 Prevention of postsplenectomy infection should include immunization against Streptococcus. pneumoniae, Haemophilus influenzae B, and Neisseria meningitides, prophylactic antibiotics, stand-by antibiotics, patient information sheets, and a medical alert bracelet.40
Conclusions
Because late effects can develop many years after cancer treatment, cancer survivors should be encouraged to perform self-examination and accept the need to be regularly monitored for late effects by physicians and centers trained on late cancer effects. The type and the periodicity of the controls depend of the cancer and the treatment received as well as cost-benefit ratio. Transplanted patients and patients who have received intensive chemotherapy should be controlled all their lives. The current understanding on late effects in cancer survivors is the foundation of recommendations and guidelines for a standardized follow-up program. These recommendations should include the type of and risk factors for late effects, the type of periodical controls to perform, the time frame, and the prophylactic and therapeutic measures to apply. In no way should the recommendations be judged as mandatory since good medical practice dictates that certain recommendations may be not applicable or contraindicated in individual patients or groups of patients. Furthermore, these recommendations have to be adapted regularly to the improvement in knowledge of late effects. Table 1 shows guidelines for the assessment of nonmalignant complications for long-term cancer survivors. The assessment includes control of the underlying disease, search of secondary malignancy, nonmalignant late effects, comprehension of the immune state and the assessment of the psychological state and quality of life. For long-term survivors after HSCT, joint recommendations on screening and preventive practices will soon be published by the European Bone Marrow Transplant Group (EBMT), Center for International Blood and Marrow Transplant Research (CIBMTR), and the American Society of Blood and Marrow Transplantation (ASBMT).
Nonmalignant late tissue and organ toxicity in adult cancer survivors.
| Organ . | Clinical manifestation . | Risk factors . | Monitoring . | Intervention . |
|---|---|---|---|---|
| Abbreviations: TBI, total body irradiation; GVHD, graft-versus-host disease; LVEF, left ventricular ejection fraction; ECG, electrocardiography; TSH, thyroid-stimulating hormone; FSH, follicle-stimulating hormone; LH, luteinizing hormone. | ||||
| Eye | Cataracts | Radiation Steroids | Split lamp examination | Fractionation of TBI Surgical repair |
| Keratoconjunctivitis | Radiation GVHD | Schirmer test | Treatment of GVHD Topical lubricants Topical steroids | |
| Heart | Restrictive or dilatative cardiopathy Arrhythmia Autonomous neuropathy | Anthracyclines Mediastinal radiotherapy | LVEF 24-hour ECG | Treatment of cardiac insufficiency Pace maker |
| Respiratory tract | Chronic obstructive lung disease Bronchiolitis obliterans | Infection GVHD Smoking | Pulmonary function testing Chest radiographic testing if clinically indicated | Treatment of infection Immune globulin substitution Treatment of GVHD |
| Restrictive lung disease | Radiation Chemotherapy Infection | Pulmonary function testing Chest radiographic testing if clinically indicated | Fractionation of radiation Lung shielding Treatment of infection Steroids | |
| Liver complications | Chronic hepatitis C | HVC infection Iron overload | Liver tests Hepatitis serologies Viral load if positive | Treatment of hepatitis C Treatment of iron overload (phlebotomy or chelation therapy) |
| Liver cirrhosis | HCV infection Iron overload | Ferritin | ||
| Chronic GVHD of the liver | Liver biopsy if indicated | Treatment of GVHD | ||
| Kidney | Nephropathy | TBI Chemotherapy (platinum) Cyclosporin | Renal function tests | Control of hypertension |
| Skeletal | Avascular necrosis of the bone | Steroids Radiation | Radiographic testing | Avoidance of long term treatment with steroids Symptomatic relief of pain Orthopedic measures Surgical repair |
| Osteoporosis | Steroids, cyclosporine, tacrolimus, hypogonadism, TBI, chemotherapy Immobilism | Dual photon densitometry | Sex hormone replacement Treatment of osteoporosis (biphosphonates, calcium, vitamin D) | |
| Oral | Chronic stomatitis | Chronic GVHD Radiation | Oral inspection | Oral hygiene Treatment of GVHD |
| Dental late effects | Radiation Chronic GVHD | Dental inspection | Prophylaxis, oral hygiene, instruction for dental care, brushing teeth, application of fluoride Treatment of caries | |
| Thyroid gland | Hypothyroidism | Radiation | TSH, T4 annually | Thyroid hormonal replacement |
| Gonadal function | Gonadal failure | Radiation Chemotherapy | FSH, LH, testosterone (males), oestradiol (females) | Hormonal replacement Sperm banking in males |
| Nervous system | Leukencephalopathy | Cranial radiation Intrathecal chemotherapy | Evaluation according to symptoms | |
| Peripheral neuropathy | Chemotherapy GVHD | |||
| Organ . | Clinical manifestation . | Risk factors . | Monitoring . | Intervention . |
|---|---|---|---|---|
| Abbreviations: TBI, total body irradiation; GVHD, graft-versus-host disease; LVEF, left ventricular ejection fraction; ECG, electrocardiography; TSH, thyroid-stimulating hormone; FSH, follicle-stimulating hormone; LH, luteinizing hormone. | ||||
| Eye | Cataracts | Radiation Steroids | Split lamp examination | Fractionation of TBI Surgical repair |
| Keratoconjunctivitis | Radiation GVHD | Schirmer test | Treatment of GVHD Topical lubricants Topical steroids | |
| Heart | Restrictive or dilatative cardiopathy Arrhythmia Autonomous neuropathy | Anthracyclines Mediastinal radiotherapy | LVEF 24-hour ECG | Treatment of cardiac insufficiency Pace maker |
| Respiratory tract | Chronic obstructive lung disease Bronchiolitis obliterans | Infection GVHD Smoking | Pulmonary function testing Chest radiographic testing if clinically indicated | Treatment of infection Immune globulin substitution Treatment of GVHD |
| Restrictive lung disease | Radiation Chemotherapy Infection | Pulmonary function testing Chest radiographic testing if clinically indicated | Fractionation of radiation Lung shielding Treatment of infection Steroids | |
| Liver complications | Chronic hepatitis C | HVC infection Iron overload | Liver tests Hepatitis serologies Viral load if positive | Treatment of hepatitis C Treatment of iron overload (phlebotomy or chelation therapy) |
| Liver cirrhosis | HCV infection Iron overload | Ferritin | ||
| Chronic GVHD of the liver | Liver biopsy if indicated | Treatment of GVHD | ||
| Kidney | Nephropathy | TBI Chemotherapy (platinum) Cyclosporin | Renal function tests | Control of hypertension |
| Skeletal | Avascular necrosis of the bone | Steroids Radiation | Radiographic testing | Avoidance of long term treatment with steroids Symptomatic relief of pain Orthopedic measures Surgical repair |
| Osteoporosis | Steroids, cyclosporine, tacrolimus, hypogonadism, TBI, chemotherapy Immobilism | Dual photon densitometry | Sex hormone replacement Treatment of osteoporosis (biphosphonates, calcium, vitamin D) | |
| Oral | Chronic stomatitis | Chronic GVHD Radiation | Oral inspection | Oral hygiene Treatment of GVHD |
| Dental late effects | Radiation Chronic GVHD | Dental inspection | Prophylaxis, oral hygiene, instruction for dental care, brushing teeth, application of fluoride Treatment of caries | |
| Thyroid gland | Hypothyroidism | Radiation | TSH, T4 annually | Thyroid hormonal replacement |
| Gonadal function | Gonadal failure | Radiation Chemotherapy | FSH, LH, testosterone (males), oestradiol (females) | Hormonal replacement Sperm banking in males |
| Nervous system | Leukencephalopathy | Cranial radiation Intrathecal chemotherapy | Evaluation according to symptoms | |
| Peripheral neuropathy | Chemotherapy GVHD | |||
Scheme of time course and relative risk of secondary leukemia and solid tumors after hemopoietic stem cell transplantation. Posttransplant lymphoproliferative disorders, which usually occur early after transplantation, are not included in the scheme.
Scheme of time course and relative risk of secondary leukemia and solid tumors after hemopoietic stem cell transplantation. Posttransplant lymphoproliferative disorders, which usually occur early after transplantation, are not included in the scheme.
University Hospital, Basel, Switzerland and Hospital Saint Louis, APHP & University Paris VII