Abstract
Peripheral T-cell lymphomas (PTCLs) are a biologically diverse and uncommon group of diseases. Compared to their B-cell counterparts, PTCLs remain largely unexplored and the optimal treatment ill-defined due to disease rarity and biological heterogeneity. For the majority of PTCL subtypes, prognosis is poor with a 5-year overall survival of approximately 30% in most series.
The notable exception is ALK-positive anaplastic large-cell lymphoma (ALK-pos ALCL), which has a superior outcome. The international prognostic index can be used to some extent to define risk groups within some PTCL subtypes, including PTCL unspecified (PTCLUS). It is likely that the observed clinical heterogeneity reflects differences at the molecular level. With the more widespread availability of gene expression profiling, it may be possible in the future to further refine the classification of PTCLs and elucidate novel therapeutic targets. Future clinical trials are needed that focus specifically on PTCL to advance our understanding and define the optimal management in this disease.
Mature T- and natural killer (NK)-cell lymphomas constitute a rare and heterogeneous group of neoplasms, the frequency varying according to geographic location and ethnic origin of the population. In the broadest description, peripheral T-cell lymphomas (PTCLs), are defined by their post-thymic origin, in comparison to precursor or pre-thymic lymphomas (e.g., lymphoblastic lymphoma). The importance of the T-cell phenotype and wide heterogeneity of the PTCL subtypes has only recently become fully appreciated. Immunophenotypic information was not utilized in the Working Formulation and, although the updated Kiel Classification recognized the T-cell phenotype, it required subclassification of PTCLs based on morphologic subtypes with poor reproducibility and failed to recognize several distinct clinicopathological entities. The REAL (Revised European-American Lymphoma) classification integrated morphologic, phenotypic, molecular, and clinical information into a unified scheme for all lymphoid neoplasms, including PTCLs, and provided the basis for the recently published World Health Organization (WHO)1 classification with several notable refinements (Table 1 ): division into predominantly leukemic, extranodal, and nodal types; separation of cutaneous and systemic anaplastic large cell lymphomas (ALCL); updated terminology for some diseases; inclusion of lymphomatoid papulosis (LyP) in the spectrum of T-cell lymphoproliferative disorders; and, recognition of subcutaneous panniculitis-type and hepatosplenic γ δ T-cell lymphomas as separate entities1 (Table 1 ).
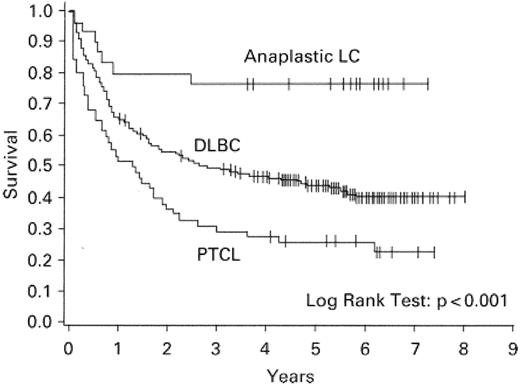
Although earlier reports, based on older classification schemes, described similar prognoses among B- and T-cell lymphomas,2 in recent years there have been several comprehensive studies that have consistently demonstrated that the T-cell phenotype imparts a negative impact on overall survival3–5 (Figure 1 ). These differences may be due to inclusion in some earlier studies of disease entities that are now appreciated to have a more favorable prognosis such as ALCL2 (Figure 1 ).
Understanding of the biology and defining the optimal treatment of PTCLs has been less well developed than that of their B-cell counterparts because of disease rarity and diversity. Frequently, dissimilar T-cell lymphomas have been combined under the term “PTCL,” potentially obscuring their distinct natural histories and limiting comparison of studies that focus on prognostic factors and survival. This review will highlight some of the more commonly encountered subtypes with a further discussion of prognostic markers and treatment strategies.
Peripheral T-cell Lymphoma, Unspecified
Peripheral T-cell lymphoma, unspecified (PTCLUS) represents the largest PTCL subtype in North America.6 In the WHO classification PTCLUS encompasses all of the PTCLs not classifiable as a specific disease entity in contrast to the rare, but “specified,” subtypes (Table 1 ). It is clear that this represents a heterogeneous group of diseases and although multiple morphologic subtypes have been recognized, evidence is lacking that these subtypes represent distinct clinicopathologic entities or have prognostic relevance. The majority of nodal cases are CD4+ and CD8−, and CD30 can be expressed in large cell variants. Most patients with PTCLUS present with nodal involvement; however, a number of extranodal sites may also be involved (e.g., liver, bone marrow, gastrointestinal [GI], skin). The majority of series report a poor outcome with a 5-year overall survival of approximately 30%–35% using standard chemotherapy.3,4,6–8
Anaplastic Large Cell Lymphoma
Anaplastic large-cell lymphoma is identified by a highly pleomorphic appearance, propensity to invade lymph node sinuses, and uniform expression of CD30 (Ki-1) on the tumor cells.9 Most are positive for epithelial membrane antigen (EMA), and more recently clusterin has been identified as a diagnostic marker of ALCL1,10 (Table 2 ). A proportion are also associated with overexpression of anaplastic lymphoma kinase (ALK), which is the result of a characteristic cytogenetic abnormality, t(2;5)(p23;q35) or an equivalent translocation, leading to overexpression of the ALK gene on chromosome 2.9 In the WHO classification it was recognized that the cutaneous form of ALCL is distinguished from the systemic type by the lack of ALK-protein expression and indolent clinical course, and thus should be considered a distinct disease entity (Tables 1 and 2 ).1
Systemic Anaplastic Large Cell Lymphoma
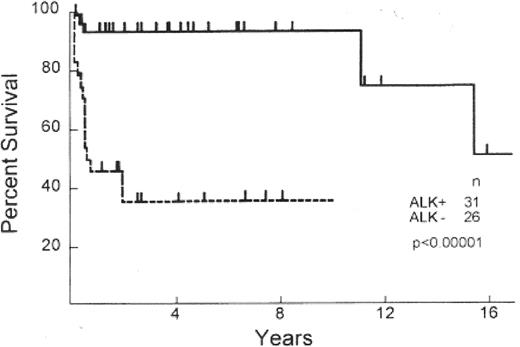
Primary systemic ALCLs display either a T- or null-cell phenotype, both harboring a clonally rearranged T-cell receptor and demonstrating a cytotoxic profile (Table 2 ).1 Systemic ALCL has a superior survival to other PTCLs, with outcomes at least comparable to, if not better than, that of diffuse large B-cell lymphoma (DLBCL)4,7,11 (Figure 1 ). However, even within this histologic subtype there is clear biological heterogeneity with a proportion of cases associated with a translocation between the ALK gene (chromosome 2) and nucleophosmin gene (NPM) (chromosome 5), resulting in the upregulation of ALK and formation of a chimeric protein (NPM-ALK). Several investigators have since demonstrated that those cases with ALK expression (~60%, ALK-positive) have a 5-year survival that is superior (93%) to those who lack expression (ALK-negative) (37%)12 (Table 2, Figure 2 ). Clinical differences are also evident between ALK-positive and -negative subtypes. ALK-negative cases often present at an older age with advanced stage, elevated lactate dehydrogenase (LDH), frequent B symptoms, and extranodal involvement.6 In fact, some investigators have pointed out that ALK-negative ALCL is more similar to PTCLUS in terms of clinical behavior and inferior survival.13
Primary Cutaneous ALCL
Primary cutaneous ALCL (CUTALCL) typically occurs in older men as solitary, asymptomatic cutaneous or subcutaneous reddish-violet nodules/tumors. Extracutaneous disease can occur in 10% of patients, mainly in regional lymph nodes, and most often in patients with multiple lesions.1 Distinguishing between CUTALCL and a closely related benign disorder, lymphomatoid papulosis (LyP), can be difficult. Spontaneous regression is the rule for LyP; however, regression also occurs in approximately 25% of CUTALCL.1 Multiple small lesions (< 1 cm) are more common in LyP, whereas primary cutaneous ALCL is typified by single or grouped lesions, often > 2 cm in size. CUTALCL should also be distinguished from systemic ALCL to avoid unnecessarily aggressive therapy (Table 2 ).
Extranodal NK/T-cell Lymphoma
Extranodal NK/T cell lymphomas, nasal-type (NK/TCL), display great variation in racial and geographic distribution and are relatively uncommon in Western populations.1 Morphologically, these tumors demonstrate angiocentric invasion, vascular destruction, and necrosis. They are positive for Epstein-Barr virus (EBV), which is thought to play a role in tumor pathogenesis. The designation “NK/T” rather than “NK” cell lymphoma is applied in the WHO classification since, although most cases appear to be NK-cell derived (CD2+, CD56+, CD3ε(cytoplasmic)+, EBV+), rare cases with identical clinical and cytologic features exhibit an EBV+CD56− cytotoxic T-cell phenotype.1 The qualifier “nasal-type” is assigned owing to the common presentation in the nasal region and associated structures; however, other identical tumors can also occur at “extra-nasal” sites such as the skin, soft tissue, GI tract, and testis.1
NK/TCLs are typically very aggressive, and despite often localized disease, the 5-year overall survival (OS) rate is only 20%–35%.14,15 Some studies have reported survivals above this range; however, inclusion of CD56− and EBV− cases or lack of reporting of immunophenotyping may have resulted in the inclusion of more favorable lymphomas.
Angioimmunoblastic T-cell Lymphoma
Angioimmunoblastic T-cell lymphoma (AILT) was initially described as an atypical reactive process in patients presenting with generalized lymphadenopathy, rash, heptato-splenomegaly, fever, and hypergammaglobulinemia.1 However, cytogenetic and molecular studies confirm clonality in the majority of cases, strongly supporting that AILT is a distinct T-cell neoplasm.1 Distinguishing AILT from PTCLUS can often be difficult; however, morphologic features favoring AILT are prominent vascularization by arborizing venules, expansion of CD21+ follicular dendritic cell networks, and the recent identification of CD10 as phenotypic marker of the neoplastic T cells.16 An oligoclonal or monoclonal B-cell population due to the expansion of B cells infected with EBV and secondary EBV+ B-cell lymphomas has been described in some patients.1 The outcome of AILT is poor, with most series reporting a 5-year OS of approximately 30% and median survival of 3 years.6
Other Uncommon PTCL Subtypes
Enteropathy type T-cell lymphoma is a rare PTCL subtype that can complicate an established history of gluten-sensitive enteropathy but most often occurs following a short history of celiac disease and/or dermatitis herpetiformis.17 Even in the absence of a known history of celiac disease, there is usually histologic evidence of villous atrophy and crypt hyperplasia in the adjacent bowel when the tumor is excised. Malignant cells are CD3+, CD7+, CD4− and are usually CD8−, but despite this, they do not express the γδ T-cell receptor. Clinically, it affects older males who present with abdominal pain and diarrhea and it may be complicated by intestinal perforation or obstruction. Although most patients have clinical stage I or IIE disease, dissemination to the liver, spleen, lung, skin, and bone marrow can occur. Treatment is often complicated by poor nutrition and significant risk of bowel perforation. Consequently, survival is extremely poor, with rare long-term survivors.6
Adult T-cell lymphoma/leukemia (ATLL) is associated with infection by the human T-cell lymphotropic virus (HTLV-1) and occurs in endemic areas of infection (e.g., Caribbean basin and southwestern Japan).17 Pathologically, the malignant cells have a distinct “cloverleaf” appearance and lack CD7, and the majority are CD4+/CD8−. The retroviral gene Tax has been shown to have a critical role in the pathogenesis of ATLL. Several clinical variants have been recognized: acute type with a rapidly progressive clinical course, bone marrow and peripheral blood involvement, hypercalcemia with or without lytic bone lesions, skin rash, generalized lymphadenopathy, hepatosplenomegaly and pulmonary infiltrates; lymphoma type with prominent adenopathy but lacking peripheral blood involvement but also associated with an aggressive course; chronic type with lymphocytosis and occasionally associated with lymphadenopathy, hepatosplenomagaly, and cutaneous lesions but having an indolent course; and smoldering type with < 5% circulating neoplastic cells, skin involvement, and prolonged survival.
Survival times in the acute and lymphomatous variants range from 2 weeks to over a year. The chronic and particularly the smoldering subtypes have a longer survival but can transform into the more acute forms. Underlying immunodeficiency associated with ATLL leads to a high rate of infections throughout the course of the disease.
Hepatosplenic γδ T-cell lymphoma (HSTCL) is a recently recognized and uncommon PTCL subtype. Most tumor cells are CD4− and CD8−, and many are associated with isochrome 7q.18 The majority of patients are young men (median age 34) who present with hepatosplenomegaly and bone marrow involvement. Rare cases of HSTCL expressing the αβ chain have been reported, but the prognostic relevance is unknown.18 The majority of patients succumb to their disease, with an overall median survival of 16 months.18 The γδ phenotype has been rarely identified in primary cutaneous T-cell lymphoma and is a marker of poor prognosis (median survival 15 months vs. 166 months in αβ subtypes).19 Unlike HSTCL, which express TIA1 but are negative for granzyme B and perforin cytotoxic proteins, primary cutaneous γδ TCL express all cytotoxic proteins, thus demonstrating an activated T-cell phenotype which may contribute to their aggressive behavior.19
Subcutaneous panniculitis-like TCL (SCTCL), is the least well-defined and rarest subtype of PTCL. The neoplastic cells are most often CD8+ αβ and express cytotoxic granules, although in 25% of cases, a γδ T-cell phenotype is identified and may be associated with a more aggressive clinical course.20 Patients often have a preceding course of waxing and waning nodules, resembling lipomas. Hemophagocytic syndrome with pancytopenia, fever, and hepatosplenomegaly has been reported in association with SCPTCL and is often a fatal complication. Durable remissions are uncommon; however, some cases can have a more indolent course.
Prognostic Factors in Peripheral T-Cell Lymphomas
Clinical factors
The International Prognostic Index (IPI) provides a prognostic score based on clinical and laboratory factors which has been validated for DLBCL and shown to predict survival accurately.21 However, insufficient immunophenotypic data were available when the IPI was designed to assess the influence of the T-cell phenotype on prognosis. Interpretation of older studies evaluating the usefulness of the IPI in PTCL are complicated by the use of outdated lymphoma classification systems and frequent “lumping” of histologic subtypes, obscuring the impact of the IPI in specific disease entities.3,4,13 However, several reports have confirmed the IPI’s usefulness in some of the specific PTCL subtypes including ALCL (ALK-positive and -negative) and PTCL US.6–8,13 Recently, the IPI has been applied to PTCLUS according to the WHO classification and found to be predictive of survival6,8 (Figure 3A ). A large study also evaluated a new prognostic model for PTCLUS which incorporates many of the current IPI factors (age, PS, LDH) in addition to bone marrow involvement.8 In this model, survival ranged from 18% (3 or 4 factors) to 62% (0 factors) (Figure 3B ). Unfortunately, many patients with PTCLUS fall into the high-risk categories with poor survival, emphasizing the urgent need for better therapies.
Attempts to use the IPI to define risk groups within NK/T-cell lymphoma have also been met with variable results.6,16 Again, heterogeneity in some analyses with inclusion of other PTCLs and, less often, B-cell lymphomas presenting in the nasal region can influence interpretation. Application of the IPI in the remaining subtypes has not been verified because the diseases are rare. However, it is unlikely that the IPI will have any clinical utility in those subtypes with an extremely poor prognosis, such as enteropathy-type and hepatosplenic γδ TCL, or in those that present almost exclusively with high-risk IPI scores.
Biological and molecular prognostic factors
PTCLUS: Attempts have been made to identify biologically distinct subgroups within the morphologically and clinically heterogeneous PTCLUS subtype. Although morphologic variants have been recognized, their prognostic significance is unknown. Most nodal cases of PTCLUS are CD4+CD8−, prompting speculation that within the CD4+, T-helper (Th) phenotype some of the biological heterogeneity may be explained by variable expression of Th1 and/or Th2 surface chemokine receptors.22 In one study, two distinct subgroups with PTCLUS were identified; group 1, positive for any of ST2(L) (Th2 marker, interleukin [IL]-1R family member), CCR5 (Th1), CXCR3 (Th1); group 2, negative for all these markers.23 Group 1 cases, considered “functional” based on the receptor expression, had a more favorable prognosis compared to group 2 cases; however, adjustment for known clinical prognostic factors was not incorporated into this analysis.23 In a separate study the prognostic significance of CXCR3 was confirmed, and CCR4 (Th2) expression was found to be associated with a poor outcome and remained significant after adjustment for other clinical factors, including the IPI.22 Interestingly, CCR4 expression correlated with CD25 expression. The functional significance of these chemokine receptor profiles remains unknown, and confirmation of their prognostic value awaits further study. It is hoped that they may provide rational targets for novel therapies in this poor-risk population.22,24
ALCL: Other biological markers beyond the ALK phenotype have been determined to have prognostic significance in ALCL. Both ALK-positive and -negative cases that express CD56 have a worse prognosis, independent of the IPI.25 Further, expression of survivin, a member of the inhibitor of apoptosis family, also confers a worse prognosis, irrespective of ALK expression.26
In ALK-negative ALCL several other biological prognostic factors have been determined to affect clinical outcome. High numbers of activated (granzyme B+) cytotoxic T-lymphocytes indicate a poor prognosis.27 Recent studies also suggest that high BCL2 protein expression in ALK-negative ALCL, a known prognostic factor in DLBCL, is associated with poor outcome.28 Conversely, expression of caspase 3, a critical component in the pro-death pathways, is associated with a favorable outcome, suggesting that alterations in apoptotic pathways may in part explain the prognostic differences between ALK-positive and -negative ALCL subtypes.28
Gene expression profiling of PTCLs
Gene expression studies have been much less extensive in the T-cell lymphomas than in B-cell lymphomas. Using the CNIO Oncochip, a recent study evaluated the gene signature of 42 newly diagnosed T-cell lymphomas that included 34 PTCLs of various histologic subtypes (including 19 PTCLUS) and compared the gene expression pattern signature to normal cellular counterparts and to lymphoblastic lymphoma.29 Within the PTCL tumors, upstream and downstream targets of the nuclear factor (NF)-κB pathway were featured in the molecular signature; however, evidence of NF-κB activation and of its effect on tumor biology await further study. This analysis was limited because it grouped all PTCL cases together, and because it mixed nodal and extranodal tumor samples; however, it is the first microarray analysis of PTCLs and will be useful for comparison in future studies. Two further groups have since attempted to utilize gene expression to subclassify PTCLs. The GELA investigators evaluated 59 primary nodal specimens of a variety of T-cell lymphomas using a cDNA array and specifically applied a multiclass predictor to separate PTCLUS into the three subgroups: U1, characterized by the expression of cyclin D2 and few reactive cells; U2, with some overlap with U1 but also associated with overexpression of genes involved in T-cell activation and apoptosis including NF-κB1 and BCL2; and U3, which included the previously defined “Lennert’s lymphoma” and was characterized by overexpression of histiocytic markers in addition to genes involved in the interferon γ/JAK/STAT pathway.30 Whether these subgroups correlate with clinical outcome awaits further study. Another group compared the gene expression profile of 17 PTCLUS nodal primary tumor specimens to normal T-cell subsets using the Affymetrix 133 A/B microarray and found that PTCLUS cells were more related to activated T cells, both CD4+ and CD8+.31 Of interest was overexpression of PDGFRA, confirmed by immunohistochemistry, which may be a potential therapeutic target.
Primary treatment approaches in PTCLs
CHOP-type chemotherapy has been the mainstay of therapy for PTCLs, but with the notable exception of ALK-positive ALCL, outcome has been uniformly disappointing. The large intergroup trial comparing CHOP to second and third generation regimens in diffuse large-cell lymphoma failed to reveal any survival differences among the regimens but unfortunately, as immunophenotyping was not widely available at the time of the study analysis, the impact of the specific treatment regimens in the subgroup of patients with T-cell lymphomas was not assessable.32 Some groups have specifically evaluated more dose-intensive regimens in PTCL6,33–35 with no firm conclusions demonstrating superiority to CHOP chemotherapy.
Generally, treatment approaches to date have been similar among the PTCL subtypes. One exception is extranodal NK/T cell lymphoma, where radiotherapy appears to be the key treatment modality, with more favorable outcomes observed in treatment regimens that incorporate radiotherapy.15,36,37 Frequent progression during anthracycline-based chemotherapy has been observed in several studies of NK/T nasal type lymphoma, suggesting an inherent resistance of these tumors to conventional systemic therapy that may be related to expression of P-glycoprotein resulting in multi-drug resistance.37 Because of this some groups have advocated the use of front-line radiotherapy in localized NK/T-cell lymphoma.37 Collectively, these results suggest that radiation should be the primary therapy in localized NK/T-cell lymphoma. However, both local and systemic relapse remain problematic, suggesting that new approaches are needed, perhaps combining radiotherapy with alternate, radiosensitizing chemotherapy. Further, survival in patients with more widespread disease at presentation is extremely poor, underscoring the need for improved chemotherapy.
Cutaneous ALCL should also be treated differently as it has an indolent course, and although relapses are frequent, the 5-year OS is approximately 90%. The majority of patients can be treated with localized excision with or without radiotherapy. Patients with disseminated skin disease may be at greater risk of developing extracutaneous involvement and may benefit from systemic therapy.1
The Role of High-Dose Chemotherapy and Stem Cell Transplant in PTCL
Several small, uncontrolled trials have attempted to evaluate the role of high-dose chemotherapy (HDC) and stem cell transplant (SCT) in PTCL. However, these studies are difficult to interpret because they are heterogeneous with respect to usage of different lymphoma classification systems; inclusion of multiple disease entities in outcome analyses; transplant type (allogeneic vs autologous); timing (primary vs relapsed/refractory); salvage and conditioning regimens used; and inclusion of pediatric cases.
Primary HDC and SCT in PTCL
With poor outcomes in most patients with PTCL using CHOP-type treatment, several investigators have attempted to add consolidation treatment for patients with PTCL, including ALCL, in first complete remission (CR1) with HDC and SCT38,39 (Table 3 ). In one study, 37 patients were transplanted in first CR with an encouraging 5-year OS of 80%.38 However, the population evaluated was young (median age 31) and included some patients with ALCL, a group with a more favorable prognosis when transplanted in CR1.40 Further, ALK protein status was unavailable.41 In ALCL, similar excellent results have been observed in other studies of up-front transplant; however, CHOP alone also results in high cure rates.12 Another prospective study evaluated 24 patients with PTCL (PTCLUS n = 12; AILT n = 12), transplanted in first remission. With a median follow-up of 15 months, 16 of the 21 patients who were successfully transplanted patients remained in remission.42 In the absence of randomized trials proving superiority to conventional chemotherapy, the use of HDC and SCT in the primary treatment remains experimental in any histological subtype.
Transplant in relapsed/refractory PTCL
Patients with relapsed PTCL who demonstrate chemosensitivity respond favorably to HDC and SCT, with long-term survival rates of approximately 35%–45%38,43,44 (Table 3 ). The results in patients with refractory PTCL are less favorable with some studies reporting no long-term survivors.38 Another report by the same authors reported a 5-year OS of 37% in a group of patients who were deemed induction failures.41 However, most of the patients had achieved a PR and in the absence of functional imaging, some of these cases may have represented true CRs.41 Success rate varies with pre-transplant IPI38 and, not surprisingly, by histologic subtype, with ALCL demonstrating consistently superior salvage rates than PTCLUS.39,43,44 These favorable results in ALCL may not extend to ALK-negative ALCL, with one small study suggesting poor outcome with HDC SCT in this population.45 Patients with relapsed or refractory PTCL and documented chemosensitive disease should be offered HDC and SCT, similar to the practice in DLBCL.
Experience with allogeneic SCT in PTCL is limited to small series with highly selected patients. Treatment mortality is high in patients receiving full myeloablative allogeneic transplant.46 Reduced-intensity conditioning (RIC) has recently emerged as an attractive strategy for patients at increased risk of treatment-related toxicity, although it has not been extensively evaluated in aggressive lymphomas. A small pilot study (n = 17) was recently performed evaluating RIC in patients with PTCL.47 The majority of cases were PTCLUS and many had relapsed after autologous HDC SCT. Although this was a highly selected population including many with a history of late relapse and almost all having a sibling donor and demonstrating chemosensitive disease, the 3-year overall and progression-free survival rates were encouraging at 81% and 64%, respectively. Further, responses were observed after donor leukocyte infusion (DLI), providing further evidence that a graft-versus-T-cell lymphoma effect may exist in PTCLs.47
Novel Treatment Approaches
For many PTCL patients current treatment strategies are largely ineffective and new therapies are being explored. Anthracycline-containing regimens have demonstrated limited success in PTCLs, with most series documenting few long-term survivors. Nucleoside analogs have recently been gaining interest in this disease. Gemcitabine has shown activity in relapsed PTCLs as a single agent,48 but has not yet been explored in combination with other agents. Compound 506U78, a pro-drug of AraG, a deoxyguanosine analog, has been evaluated as a single agent in PTCL. The drug is rapidly converted in the blood to Ara-G and has toxic effects on T-cells. Unfortunately, grade 3/4 neurotoxicity is problematic, and early treatment related deaths have limited further development.49
IL2-receptor (IL-2R) is a marker of T-cell differentiation, and the CD25 subunit of this receptor is expressed in a subset of patients with PTCLUS and CD30+ ALCL. Denileukin diftitox (ONTAK) is a fusion molecule of IL-2 to diphtheria toxin that targets tumor cells expressing the IL-2R and has been shown to have efficacy in patients with cutaneous T-cell lymphoma. Limited reports have also demonstrated tumor responses in relapsed PTCLs50 (Table 4 ), but toxicity remains significant. Flu-like symptoms and hypersensitivity reactions have limited its usage and combination with other therapies. Antibodies directed at the CD25 subunit (human anti-TAC antibody, daclizumab) and an anti-CD25 recombinant immunotoxin in addition to a radioconjugate of anti-Tac with 90-yttrium are also currently being evaluated as potential therapeutic agents.51
Histone deacetylase inhibitors (HDIs) are a new class of anti-neoplastic agents currently being evaluated in cancer clinical trials. Histone acetylation modulates gene expression, cellular differentiation, and survival and is regulated by the opposing activities of histone acetyltransferases (HATs) and histone deaceylases (HDACs). HDAC inhibition results in accumulation of acetylated nucleosomal histones and induces differentiation and/or apoptosis in transformed cells. Depsipeptide, an inhibitor of histone deacetylation, has demonstrated activity in relapsed PTCL patients with a overall response rate of 26%.52
Recently, immunotherapy has emerged as an important adjunct to traditional cytotoxic chemotherapy in B-cell lymphomas. Alemtuzumab, a humanized monoclonal antibody directed against the CD52 antigen, is present on most malignant T-cells, making it an attractive target for PTCL. Additional widespread CD52 expression on normal T/B-cells, monocytes, and macrophages results in profound immunosuppression and has restricted combination with chemotherapy. As a single agent in heavily pretreated patients with PTCL the overall response rate with alemtuzumab was 36% in one report.53 Several studies are evaluating the addition of alemtuzumab with chemotherapy. Combination of alemtuzumab with fludarabine, cyclophosphamide, and doxorubicin in untreated and relapsed patients was evaluated in a variety of PTCLs.54 Of 18 evaluable patients, the majority had PTCLUS (n = 10), with an overall response rate of 61%, and those patients treated primarily with this regimen had a CR rate of 78%; however, assessment of survival awaits longer follow-up. Of concern, over half of the patients developed cytomegalovirus (CMV) reactivation. These encouraging results suggest that the combination of alemtuzumab with chemotherapy is feasible; however, toxicity remains a major limitation.
CD30 is uniformly expressed in ALCL and a subset of patients with PTCLUS. With minimal expression on normal cells, it is the ideal therapeutic target. Phase I/II studies have been initiated with chimeric (SGN30) and fully humanized antibodies directed against CD30 (MDX-060, 5F11) in ALCL55,56 (Table 4 ). Several objective responses have been reported in phase II reports, and ongoing studies with these agents suggest that an additive benefit is seen in combination with chemotherapy. Unfortunately, the majority of PTCLs do not express this antigen and better targets, with limited expression on normal tissue, are needed. With recent studies demonstrating that a subset of patients with PTCLUS and ATLL express CCR4, investigators are evaluating the efficacy of anti-CCR4 monoclonal in these diseases.57 Finally, studies are also underway testing the effect of tumor vaccines directed against the idiotypic TCR, particularly in cutaneous TCLs, and targeting the ALK protein in ALK+ ALCL.58
In summary PTCLs represent a heterogenous group of diseases, most of which have disappointing cure rates. Therapeutic progress has been slow due to disease rarity and inclusion in studies focused on B-cell NHL. With the development of microarray technology, the molecular heterogeneity of these diseases, particularly the very diverse subgroup PTCLUS, may be better understood and provide clues as to new biological subtypes and highlight novel therapeutic targets. Future studies should focus on PTCLs in isolation, preferably as individual disease entities due to their unique clinical behavior, an effort that will require multi-institution collaboration.
Survival of patients with peripheral T-cell lymphoma (PTCL) compared with anaplastic large cell (LC) lymphoma and diffuse large B-cell lymphoma (DLBC).
Reproduced with permission from Armitage JO, Vose JM, Weisenburger DD,59 with permission from Oxford University Press.
Survival of patients with peripheral T-cell lymphoma (PTCL) compared with anaplastic large cell (LC) lymphoma and diffuse large B-cell lymphoma (DLBC).
Reproduced with permission from Armitage JO, Vose JM, Weisenburger DD,59 with permission from Oxford University Press.
Survival of T-cell/null cell anaplastic large cell lymphoma (ALCL) patients by anaplastic lymphoma kinase (ALK) expression.
Originally published in Blood. Reproduced from Gascoyne RD, Aoun P, Wu D, et al12 with permission from the American Society of Hematology.
Survival of T-cell/null cell anaplastic large cell lymphoma (ALCL) patients by anaplastic lymphoma kinase (ALK) expression.
Originally published in Blood. Reproduced from Gascoyne RD, Aoun P, Wu D, et al12 with permission from the American Society of Hematology.
A: Overall survival of peripheral T-cell lymphoma, unspecified (PTCLUS) according to the International Prognostic Index; B: Overall survival of PTCLUS according to proposed new prognostic index (age > 60, performance status > 2, elevated LDH, bone marrow involvement).
Low risk: 0, 1, 5 y OS 59%; low-intermediate risk: 2, 5 y OS 46%; high-intermediate risk: 3, 5 y OS 40%; high-risk: 18%; P < .0001. Group 1: 0 factors, 5 y OS 62%; group 2: 1 factor, 5 y OS 53%; group 3: 2 factors, 5 y OS 33%; group 4: 3 or 4 factors, 5 y OS 18%; P < .0001.
Abbreviations: LDH, lactate dehydrogenase.
Originally published in Blood. Reprinted from Gallamini A, Stelitano C, Calvi R, et al8 with permission from the American Society of Hematology.
A: Overall survival of peripheral T-cell lymphoma, unspecified (PTCLUS) according to the International Prognostic Index; B: Overall survival of PTCLUS according to proposed new prognostic index (age > 60, performance status > 2, elevated LDH, bone marrow involvement).
Low risk: 0, 1, 5 y OS 59%; low-intermediate risk: 2, 5 y OS 46%; high-intermediate risk: 3, 5 y OS 40%; high-risk: 18%; P < .0001. Group 1: 0 factors, 5 y OS 62%; group 2: 1 factor, 5 y OS 53%; group 3: 2 factors, 5 y OS 33%; group 4: 3 or 4 factors, 5 y OS 18%; P < .0001.
Abbreviations: LDH, lactate dehydrogenase.
Originally published in Blood. Reprinted from Gallamini A, Stelitano C, Calvi R, et al8 with permission from the American Society of Hematology.