Abstract
New discoveries in cell biology, molecular biology and genetics have unveiled some of the pathophysiological mysteries of some of the bone marrow failure syndromes. Many of these discoveries have revealed why these syndromes show so much clinical overlap and some hold the potential for influencing the development of new therapies. In children and adults with pancytopenia and hypoplastic bone marrows proper differential diagnosis requires that some attention be directed toward defining molecular and cellular pathogenetic mechanisms because, once identified, some of these mechanisms will clearly suggest rational therapeutic approaches, treatment options that should be avoided, or both.
In Section I, Drs. Jeffrey Lipton and Grover Bagby review the approach to diagnosis and management of patients with the inherited bone marrow failure syndromes, Fanconi anemia, dyskeratosis congenita, Diamond-Blackfan anemia, and the Shwachman-Diamond syndrome. Extraordinary progress has been made in identifying the genes bearing pathogenetically relevant mutations in these disorders, but slower progress has been made in defining the precise functions of the proteins these genes encode in normal cells, in part because it is increasingly obvious that the proteins are multifunctional. In practice, it is clear that in patients with dyskeratosis congenita and Fanconi anemia, the diagnosis must be considered not only in children but in adults as well.
In Section II, Dr. Elaine Sloand outlines a very practical and evidence-based approach to diagnosis and management of acquired hypoplastic states emphasizing overlap between non-clonal and clonal hematopoiesis is such conditions. The pathogenesis of T lymphocyte–mediated marrow failure is presented as a clear-cut rationale for use of immunosuppressive therapy and stem cell transplantation. Practical management of patients with refractory disease with and without evidence of clonal evolution (either paroxysmal nocturnal hemoglobinuria [PNH] or myelodysplasia [MDS]) is presented.
In Section III, the challenge of hypoplastic MDS is reviewed by Dr. Charles Schiffer. After reviewing the most up-to-date classification scheme, therapeutic options are reviewed, focusing largely on agents that have most recently shown some promising activity, including DNA demethylating agents, thalidomide and CC5013, arsenic trioxide, and immunosuppressive therapy. Here are also outlined the rationale and the indications for choosing allogeneic bone marrow transplantation, the only therapy with known curative potential.
I. Inherited Aplastic Anemias
Grover C. Bagby, MD, and Jeffrey M. Lipton MD, PhD*
Albert Einstein College of Medicine; Pediatric Hematology/Oncology & Stem Cell Transplantation, Schneider Children’s Hospital, 269-01 76th Avenue, Rm 255, New Hyde Park NY 11040
As the genetic basis and molecular pathogenesis of many of the inherited marrow failure syndromes are identified, it has become clear that some of these disorders may present as bone marrow failure or myelodysplasia (MDS) as well as epithelial malignancies well into adulthood. Distinguishing inherited aplastic anemia from acquired is not simply an esoteric exercise. The particular diagnosis will have an impact on proper management strategies and therapeutic approaches. Many of the rare inherited marrow failure syndromes described in Table 1 may result in pancytopenia; only four will be reviewed below. We have chosen these in large part because their genetics and biochemical mechanisms are better characterized than the others. We will not discuss Kostmann syndrome (severe congenital neutropenia), in which pancytopenia occurs only very rarely; congenital amegakaryocytic thrombocytopenia; or the very rare disorders congenital dyserythropoietic anemia, Dubowitz and Seckel syndromes, Pearson syndrome, cartilage-hair hypoplasia or reticular dysgenesis. For more information regarding these entities we refer the reader to a recently published comprehensive review.1
Disorders Frequently Associated with Multilineage Bone Marrow Failure
Two disorders classically present with aplastic or hypoplastic bone marrow failure involving all hematopoietic lineages: Fanconi anemia (FA) and dyskeratosis congenita (DC). Both of these disorders are associated with a high risk of MDS and acute nonlymphocytic leukemia (AML), and, when adults with these disorders are diagnosed, they commonly present with either hematopoietic (MDS/AML) or epithelial neoplasms (Table 2 ). Because the initial diagnosis of both of these disorders has been made in individuals in their 5th and 6th decades of life, they must be considered even in adults. In particular, both of these diseases should be considered in any young adult with any one of the following: subtle but characteristic physical anomalies, hematologic cytopenias, unexplained macrocytosis, MDS/AML, or squamous cell cancer even in the absence of severe pancytopenia or a positive family history.
Fanconi Anemia
Clinical and hematologic features:
Fanconi anemia is an autosomal recessive disease characterized by bone marrow failure, developmental anomalies (e.g., absent thumbs, absent radius, microcephaly, renal anomalies), short stature, abnormal skin pigmentation (café au lait and hypo- or hyperpigmented spots), a high incidence of MDS and AML and epithelial malignancies later in life, and cellular hypersensitivity to crosslinking agents. As many as half of patients with FA may not exhibit obvious developmental or skin abnormalities, and it is increasingly clear that the diagnosis should be considered in adults with bone marrow failure, MDS, or early onset epithelial malignancies. However, apart from a few of those identified because they were siblings of a newly diagnosed FA patient, virtually all newly diagnosed FA patients have abnormal blood counts (initially thrombocytopenia and macrocytosis) and most have pancytopenia.
Genetics and molecular pathogenesis:
All racial and ethnic groups are at risk, and 11 or more complementation groups are known to date. One in 300 persons in Europe and the United States are heterozygotes.1 Genes for 8 groups have been characterized (FANCA, C, D2, E, F, G, L, and BRCA2) (reviewed in 2,3). FANCA is the most common complementation group (Table 3 ).
One clear function of the FA proteins is to maintain chromosomal stability but it is not yet clear how this is accomplished. What is known is that 5 or 6 of the 8 known FA proteins (FANCA, -C, -F, -G, -L and possibly FANCE) bind together in a nuclear complex. This complex may have many functions but so far it is only clear that it can influence the capacity of a seventh, FANCD2, to co-localize with BRCA1 and BRCA2 in “nuclear foci” following genotoxic stress.2,3 This colocalization response requires that FANCD2 be monoubiquitinylated, and monoubiquitinylation is permitted only if the FA core complex is intact. Inactivating mutations of any one of the FA proteins in the complex disrupts the complex and prevents FANCD2 monoubiqutinylation. The ubiquitin ligase that performs this function is unknown but may be FANCL, a newly described FA protein that exhibits general ubiquitin ligase capacity. Carboxy-terminal truncating mutations of the seventh FA gene, BRCA2, are hypomorphic and lead to FA-D1. The intersection of the BRCA1/2 and FA pathways has led to increasing interest in the function of the FA “pathway” in sporadic malignancies. However, despite the very clearly interesting and dynamic protein-protein interactions, the functions of the FA proteins in the nucleus are unknown at a biochemical level. Thus, there is not yet a clear biochemical function of the FA nuclear pathway, but when it is defective cellular responses to genotoxic stress are deficient, at least in nontransformed cells.
It is unlikely that the nearly universal finding of marrow hypoplasia is related simply to intolerance of crosslinking agents or general cytogenetic instability; otherwise, other organ systems should fail as much as hematopoietic tissues. In fact, most FA cells are also intolerant of oxidative stress and at least one of the proteins (FANCC) is clearly involved in survival signaling and in modulating responses to apoptotic cytokine cues.2 Probably as a result of loss of these additional functions of FA proteins, bone marrow progenitor cells and stem cells are pro-apoptotic in FA patients.2 In fact, there is some evidence in humans and mice that the combination of genetic instability (loss of the nuclear function of FA proteins) and apoptotic hematopoietic stem cells (loss of the signaling functions of FA proteins) provides a selective force for the evolution of adapted hematopoietic stem cell clones that lead to leukemia and MDS.2 Therefore, some argue that all the FA proteins will prove to be multifunctional, each having an impact on genetic stability and each enhancing stem cell survival. Furthermore, the accelerated apoptosis of hematopoietic stem cells and progenitors and a predisposition to myeloid leukemia is, to a varying degree, a consistent finding in the inherited bone marrow failure syndromes.4 This suggests that protein multifunctionality may be a common theme.
Diagnosis:
Classic clinical features such as growth retardation, small head size, café-au-lait spots, and radial ray defects can be strong diagnostic clues, but Fanconi anemia can occur in patients without congenital defects and can be ascertained in adulthood. Increases of HbF and macrocytosis are commonly noted but their absence cannot rule out the disease. Therefore, the safest operating principle is to consider this disease in all young adults and children with hypoplastic or aplastic anemia or cytopenias, unexplained macrocytosis, MDS, AML, epithelial malignancies or subtle but characteristic physical anomalies. In the proper clinical context the gold-standard screening test for Fanconi anemia is based on the characteristic hypersensitivity of FA cells to the crosslinking agents (mitomycin C, diepoxy butane [DEB], cisplatin). Culture of replicative cells (usually phytohemagglutinin [PHA]-stimulated peripheral blood lymphocytes or skin fibroblasts) in the presence of low doses of either mitomycin C (MMC) or DEB followed by examination of metaphase spreads for evidence of chromosomal breaks and radial chromosomes2 can establish the diagnosis of FA. Mutated genes can be identified by retroviral complementation studies, by direct sequencing, or by denaturing high performance liquid chromatography (DHCLP) heteroduplex analysis.
Management:
The median survival of patients with FA is approximately 30 years but survival is extraordinarily variable.5 The most life-threatening early event in most complementation groups is bone marrow failure (patients with homozygous BRCA2 mutations, who seem to have early onset epithelial malignancies, may be exceptions), so management of bone marrow failure is the primary concern. Stem cell transplantation is the only option for establishing normal hematopoiesis. For a more comprehensive discussion of hematopoietic stem cell transplantation (HSCT) for the inherited bone marrow failure syndromes readers are referred to a recent comprehensive review.6 There is general agreement that an otherwise healthy patient with FA and significant pancytopenia (absolute neutrophil count < 1000/mm3, hemoglobin ≤ 8g/dL or a platelet count ≤ 40–50,000/mm3) and an available HLA-matched sibling donor is an excellent candidate for hematopoietic stem cell transplantation. Given that these patients are extraordinarily sensitive to the chemotherapeutic agents and radiation ordinarily used to condition recipients, the doses of conditioning agents must be reduced to avoid fatal toxicities. Even with such reductions, there is some retrospective evidence that long-term survivors are at high risk of epithelial cancers of the head and neck. This has prompted studies on the use of nonmyeloablative preparative regimens for transplant. Five-year survival of patients receiving stem cells from HLA-identical siblings approaches 75% and in some centers 5-year survival is 58% with matched unrelated stem cell donors.7 All probands and siblings should be HLA-typed early and sibling cord blood samples should be preserved. In vitro fertilization and pre-implantation genetic diagnosis (both to rule out FA and rule in an HLA match) has been used successfully, and in one instance the cord blood stem cells of the sibling were used successfully to transplant the proband.8
Clearly not all patients are candidates for transplantation. Apart from supportive measures, androgen therapy frequently induces meaningful responses in pancytopenic patients (reviewed in 1) but androgens are usually reserved for transfusion-dependent patients or patients with platelet counts and neutrophil counts that put them at high risk for bleeding and infection. Gene therapy for FA patients is a theoretically appealing option but is currently not validated. New clinical trials of stem cell gene therapy for patients with FANCA or FANCC mutations are expected to open before January 2005. For patients with stable disease, annual surveillance exams and bone marrow aspiration (with cytogenetic studies) and biopsy are suggested but not evidence-based. For patients with complex cytogenetic abnormalities or MDS, closer follow-up is warranted.
Finally, the treatment of malignancies that develop in patients with Fanconi anemia is extraordinarily difficult, particularly those for which the standard of care is either radiation, alkylating agents (e.g., cisplatin) or both. If FA patients are treated with full doses of alkylating agents or radiation the hypersensitivity of FA cells to such treatment will result in extraordinary morbidity and mortality. Treatment of patients with such malignancies should be carried out in collaboration with specialized centers.
Dyskeratosis congenita
Clinical and hematologic features:
DC, a rare bone marrow failure syndrome first described in 1906, is attended by the classic triad of abnormal skin pigmentation, nail dystrophy, and oral leukoplakia. In addition to these defining lesions other common somatic abnormalities include epiphora (tearing secondary to obstructed tear ducts), developmental delay, pulmonary disease, short stature, esophageal webs, dental caries, tooth loss, premature grey hair and hair loss. The skin findings can range from tan macular or reticular hyperpigmentation to hypopigmented macular lesions. The typical location of such lesions is on the sun-exposed areas of the upper trunk, face and arms. Mucosal leukoplakia is almost always in the oropharynx but other aerodigestive and urogenital sites can be involved. Commonly these mucosal abnormalities occur in the second, third or fourth decade of life. Nail dystrophy usually involves the fingernails before the toenails are involved. Dystrophy is characterized by longitudinal fissures, atrophy and, later, by nail distortion and, in some cases, nail loss.
The Dyskeratosis Congenita Registry (DCR), established in 1995, has provided valuable data regarding epidemiology, pathophysiology, genetics and treatment of DC. In a recently published report9 there were 148 patients from 92 families emanating from 20 countries enrolled in the DCR. The median age for the onset of mucocutaneous abnormalities in patients enrolled in the DCR is 6–8 years. Nail changes occur first. Pancytopenia is the hematologic hallmark of DC. The median age for the onset of pancytopenia is 10 years. Approximately 50% of patients reported in the literature develop severe aplastic anemia and more than 90% of individuals reported in the DCR have developed at least a single cytopenia by 40 years of age.9 In a number of cases aplastic anemia preceded the onset of abnormal skin, dystrophic nails or leukoplakia. As with FA it is the nonhematologic manifestations of DC that are of particular concern when hematopoietic stem cell transplantation for bone marrow failure is contemplated.
The clinical course is highly variable, even within families. In the less severe forms, clinical manifestations may not be present at the time of birth but evolve during early childhood and adolescence. In the X-linked form one third develop bone marrow failure as teenagers as do 60% of those with the autosomal recessive form.1 Yet, while most patients have hypoplastic bone marrows at the time of diagnosis, ascertainment of cases does occur in adulthood and such cases, as is the case with FA, can present with MDS (generally hypoplastic), AML, or epithelial malignancies.9 A very severe variant of the X-linked syndrome, known as the Hoyeraal-Hreidarsson syndrome, involves early onset bone marrow failure, intrauterine growth retardation, microcephaly, cerebellar hypoplasia, mental retardation, and immune deficiency.9
Epithelial malignancies develop at or beyond the third decade of life. About 1 in 5 patients will develop progressive fibrotic pulmonary disease resulting in diminished diffusion capacity and/or restrictive lung disease. It is likely that more pulmonary disease would be evident if patients did not succumb earlier to the complications of severe aplastic anemia and cancer. The Registry has, somewhat surprisingly, revealed the presence of significant progressive immunodeficiency in DC. The vast majority (80%) of patients who died, with or without significant neutropenia, did so from infection, some opportunistic, usually before 30 years of age. Over half of the patients studied had a predominantly cellular immune defect, and it is therefore reasonable to assume that immunodeficiency as well as neutropenia plays a significant role in infectious morbidity and mortality in DC.9
Genetics and molecular pathogenesis:
DC may display an autosomal dominant (OMIM 127550) and recessive (OMIM 224230) as well as X-linked (OMIM 305000) pattern of inheritance. About 10% of DC patients have the autosomal dominant form of the disease,1 now known to be associated with mutations in the telomerase RNA gene hTERC.10 This form of the disease is likely the result of haploinsufficiency and is less severe than the X-linked form. It is, however, characterized by “disease anticipation” in which the onset of disease occurs at increasingly earlier ages in successive generations.11 Individuals heterozygous for TERC mutations may be asymptomatic well into the sixth decade of life, but this asymptomatic phase is likely to be shorter in each successive generation of heterozygotes.11 About 80% of DC patients have the X-linked form of the disease1 caused by mutations in the DKC1 gene (Xq28) encoding the conserved 58 kDa nucleolar protein dyskerin.12 That this protein is known to associate with TERC RNA is intriguing and suggests that the molecules interact to determine telomerase function. However, confirmatory biochemical studies need to be done to prove this point because nucleolar proteins also participate in ribosomal biogenesis and responses to cellular stress, so dyskerin may have an entirely separate function unrelated to telomerase.
Diagnosis:
Screening for the X-linked and autosomal dominant forms of DC should be considered in children and adults who have (1) bone marrow failure, AML or MDS; (2) negative mitomycin C and DEB tests (to rule out FA); and either (3) hypopigmented macules, reticulated hyperpigmentation, dystrophic nails, or oral leukoplakia or (4) evidence in the family history of either X-linked or autosomal dominant bone marrow failure, MDS, or AML, particularly if there is evidence of “disease anticipation.” Patients with the X-linked and autosomal dominant forms of DC can be diagnosed by sequencing of genomic DNA. For TERC, the entire coding region is in exon 1 and mutations include large and small deletions, single base changes, and missense mutations. DKC1 screening involves sequencing of 15 exons, and mutations include missense (the majority), splice site mutations, a large deletion, and a promoter mutation. Large deletions of DKC1 may be missed by using sequence analysis in carrier females and may also be missed in the TERC gene.
Management:
Sixty-seven percent of the deaths reported in the DC appear to be a consequence of bone marrow failure; therefore, therapeutic focus is on transfusion support and measures targeting hematopoietic activity. Androgen therapy, granulocyte colony-stimulating factor (G-CSF) and granulocyte-macrophage colony-stimulating factor (GM-CSF) have been used with some temporary successes. But the role of such agents in management of DC has not been as clearly demonstrated as it has in patients with FA. Nonetheless, the use of G-CSF or GM-CSF cannot be avoided in patients whose neutropenia has proven to be severe and life-threatening.
Almost 9% (13/148) of patients developed cancer. Of these, 4 patients had MDS, 1 had Hodgkin disease and 8 had carcinoma. The relative risk of leukemia has not yet been defined. Stem cell transplantation is an acceptable approach, but there are unexplained post-transplant morbidities6 that warrant use of this approach only in the context of a clinical trial. For example, 9% of patients with DC died of lung disease with or without HSCT. It is prudent to assume that all DC patients are at a high risk of interstitial pulmonary disease when undergoing HSCT, and it is now recommended that conditioning regimens take this into account by avoiding lung radiation (by shielding) and avoiding chemotherapeutic agents known to have pulmonary toxicities. As is the case in FA patients, nonmyeloablative conditioning regimens may reduce the incremental risk of pulmonary toxicity as well as the hypothesized incremental nonhematologic cancer risk.13 Unfortunately, there have been too few transplant survivors to determine whether an increase in the prevalence of cancer will follow as a consequence of HSCT. Stem cell gene therapy or stem cell telomere engineering are rational future considerations. As in patients with FA, surveillance bone marrow studies are generally performed annually unless there is myelodysplasia or a complex clonal cytogenetic abnormality, in which case more frequent follow-up may be warranted.
Inherited Diseases Associated with Failure of Single Hematopoietic Lineages
Certain inherited marrow failure states present in the first year of life with significant reductions of either the erythropoietic (e.g., Diamond-Blackfan anemia [DBA]) or granulopoietic (e.g., Shwachman-Diamond syndrome [SDS]) marrow. These disorders only occasionally evolve to hypoplastic bone marrows and pancytopenia. For this reason only rare patients may be ascertained in the later pancytopenic stages. As in the case with DC and FA, clonal evolution to MDS and AML has been described in both DBA and SDS. The distinguishing features of these diseases vis-à-vis DC and FA is that they rarely present in adulthood and most often begin with a failure of a single hematopoietic lineage.
Diamond-Blackfan anemia
Clinical and hematologic features:
DBA (OMIM #205900) is a rare pure red cell aplasia with a frequency of 2–7 per million live births, usually presenting in early infancy and with little if any suppression of other hematopoietic lineages.
The Diamond-Blackfan Anemia Registry (DBAR) of North America, established in 1993, has provided demographic, laboratory and clinical data on DBA patients in the United States and Canada.14 The median age at presentation of anemia is 2 months and the median age at diagnosis of DBA is 3 months. Over 90% of the patients present during the first year of life. Physical anomalies, excluding short stature, were found in 47% of the patients in the DBAR. Of these, 50% were of the face and head, 38% upper limb and hand, 39% genitourinary and 30% cardiac. Patients with multiple anomalies within each of the above four categories were considered as having a single anomaly within that category. Using these criteria, more than one anomaly was found in 21% of the patients.
Elevated erythrocyte adenosine deaminase (eADA) activity found in approximately 85% of patients, macrocytosis, and an elevated fetal hemoglobin are supportive but not diagnostic of DBA. These parameters have been helpful in distinguishing DBA from transient erythroblastopenia of childhood (TEC), a self-resolving hypoplastic anemia, thus avoiding unnecessary treatment.
The recognition of DBA as a cancer predisposition syndrome, although not to the same extent as is the case with FA and DC, further complicates management decisions. In a recent report there were 6 of 354 evaluable patients in the DBAR with malignancy. Three patients had osteogenic sarcoma; 1, MDS; 1, colon carcinoma; and 1, a soft tissue sarcoma.14 A review of the literature revealed 23 additional cases of cancer. Among these were 10 cases of AML/MDS, 4 lymphoid malignancies, 2 cases of osteosarcoma, 2 breast cancers and 5 other cancers.14 Furthermore instances of significant cytopenias, including aplastic anemia, are emerging.14
Genetics and molecular pathogenesis:
Mutations in the RPS19 (19q1.32) gene have been found to underlie DBA in about 25% of patients studied to date. Other DBA genes exist, one located on 8p23.2 and another not yet identified.15 Dominant and recessive forms of DBA have been reported, but 50%–60% of cases with RPS19 mutations are de novo and sporadic.16 The precise function of the 16 kDa nucleolar protein RPS19 (OMIM *603474) is unknown but its nucleolar localization is disrupted by disease-causing mutations, and retroviral expression of RPS19 in progenitor cells from DBA patients with RPS19 mutations enhances erythropoiesis.17 There is recent direct evidence in studies on knockout mice that complete deficiency of RPS19 is lethal.18
Diagnosis:
Screening for the RPS19 mutations involves sequencing each of the 5 coding RPS19 exons. However, diagnosis in the majority of DBA patients is made on clinical grounds because only the minority (25%) will have mutations in RPS19. Thus the disease is considered likely in children with (1) normocytic or macrocytic anemia associated with reticulocytopenia and bone marrow erythroblastopenia, and (2) no evidence of FA by chromosomal breakage testing. Elevated erythrocyte ADA activity is a common feature and can be diagnostically helpful.14 Diagnosis in adults is uncommon, but more and more cases are being ascertained in family studies of afflicted children. There is no doubt that there are cases of mild anemia and unexplained macrocytosis as well as individuals with characteristic birth defects who have unrecognized DBA.
Management:
Corticosteroid therapy is a key element in the management of children with DBA. In large series from the European registry representing France and Germany and the DBAR,19 63% and 80% initially responded to steroid therapy, respectively. The natural course of the disease in steroid-treated patients is unpredictable. In fact, responses can range from rapid increases in reticulocyte counts within 1–2 weeks followed by a long period of steroid independence, to complete unresponsiveness to corticosteroids. Due to significant side effects (poor growth, pathological fractures and cataracts), only approximately 40% of steroid-responsive patients will remain on corticosteroids at an acceptable every other day dose. Indeed, the current recommendation is to withhold steroids until after the first year of life in order to protect growth during this critical period as well as permit live viral vaccinations. Twenty percent of patients enter a remission and discontinue either corticosteroids or transfusion therapy and their long-term prognosis is excellent. Patients who can be maintained with low doses of steroids also do quite well.1,14
Because stem cell transplantation has been successful in the management of steroid-resistant transfusion-dependent or pancytopenic patients, it is recommended that all probands and siblings be HLA-typed early and that sibling cord blood samples be preserved. Recipients of matched sibling donors have an actuarial survival of about 80%.6,14
Shwachman-Diamond syndrome
Clinical and hematologic features:
Described simultaneously in the United States and in Great Britain in 1964, SDS is an autosomal recessive disorder (OMIM 260400) characterized by bone marrow failure (classically neutropenia), exocrine pancreatic insufficiency and metaphyseal dysostosis. There is also an increased relative risk of MDS and AML.20 One of the two most common causes of pancreatic insufficiency in children, this disorder ordinarily presents in early childhood as failure-to-thrive. It can be associated with other congenital anomalies, prominently skeletal (rib and thoracic cage abnormalities), short stature, delayed puberty, developmental delays and learning disabilities, ichthyosis, liver dysfunction, dental caries and dysplastic teeth. Interestingly, while pancreatic insufficiency can improve later in life,20 bone marrow failure persists and can be later accompanied by clonal evolution to MDS and AML. Hypoplastic granulopoiesis is the most reliable hematologic abnormality. In a series of 88 patients, 86 had neutropenia, only 36 (of 86) had anemia, 28 of 82 had thrombocytopenia, and pancytopenia was present in only 16 of 85.21 Bone marrow biopsies are typically hypocellular. MDS was present in 10% and clonal abnormalities in 14%, but the lifetime risk of MDS/AML is unknown. There are reports of clonal cytogenetic abnormalities occurring in marrow cells resembling those seen in FA, commonly involving chromosome 7 but including chromosomes 1, 9, and 20.22 As has been described anecdotally in patients with FA, some clonal chromosome abnormalities disappear over time. Naturally, the ascertainment of this type of cytogenetic information is on a macroscopic scale, so whether the mutant stem cell is entirely gone for good or merely quiescent (in which case its progeny would not be ascertained in the bone marrow sample but might reappear again later) is not yet known. It is fair to say that, in this disease, to date there is no clear prognostic value in the discovery of only one or two cytogenetic defects in a hematopoietic clone.22
Genetics and molecular pathogenesis:
SDS is caused by inactivating mutations of the SBDS gene located on chromosome 7.23 This highly conserved gene is widely expressed in most human cell types and encodes a 250 amino acid protein the function of which is unclear, although indirect genetic evidence suggests that SBDS may be involved in RNA processing.23,24 Mutant forms of other rRNA processing proteins are associated with other marrow failure syndromes, including DC and cartilage-hair hypoplasia. Whether the RNA-processing defect is directly linked with the hematopoietic or pancreatic phenotype is not yet known, and it is important that scientists working in the field evaluate potential non-RNA functions of the protein.
Diagnosis:
For selectively neutropenic children, evidence of pancreatic insufficiency (low serum trypsinogen and isoamylase, abnormal 72-hour fecal fat content) should clearly suggest the diagnosis. Patients may have fatty infiltration of the pancreas on sonogram, MRI or CT scans. For patients presenting with bone marrow failure, FA, DC and even DBA should be excluded. Genetic testing for SBDS mutations can be performed on DNA from buccal swabs or blood (blood as a source of DNA cannot be used if patients have had a stem cell transplant). Such testing involves bidirectional sequencing of the exons and intron/exon boundaries of exons 1 through 5 of the SDBS gene on chromosome 7q11. Seventy-five percent of the alleles associated with SBDS represent gene conversion mutations involving an SBDS pseudogene. Ninety percent of patients carry at least one converted allele and 60% carry two. The majority of conversion events involve exons 2 and 3. Prenatal diagnosis is available.
Management:
Supportive care, pancreatic enzyme replacement, G-CSF for severe neutropenia, and matched sibling stem cell transplantation are current standards of care for SDS. Because the disease is rare, the rationale for each of these approaches is empirical. As with FA and DC, somatic cell intolerance has resulted in poor transplant outcomes. Thus, transplant should be carefully considered on a case-by-case basis using carefully designed attenuated conditioning regimens. Regular hematologic, gastrointestinal, and orthopedic follow-up is required to monitor evolving clones, resolution of pancreatic insufficiency, and development of hip and knee dysfunction, respectively.
II. Acquired Aplastic Anemia
Elaine M. Sloand, MD*
National Heart, Lung, and Blood Institute, National Institutes of Health, 1600 North Oak Street, #1820, Arlington VA 22209
Acquired aplastic anemia (AA) is a disease characterized by pancytopenia and a hypocellular bone marrow. The disease preferentially affects young adults and individuals over the age of 60 years.1
Clinically, AA should be distinguished from other conditions that lead to pancytopenia as well as from congenital forms of bone marrow failure including FA. A family history of aplasia when present is strongly suggestive of a constitutive bone marrow failure syndrome, but may be negative in recessively inherited disorders (reviewed in 2). Determining whether a patient has AA or hypoplastic MDS may be difficult and sometimes arbitrary in the absence of cytogenetic abnormalities. This distinction may not be critical as substantial overlap exists between MDS and AA; younger patients with hypoplastic MDS of recent onset may be as likely to respond to immunosuppression as patients with AA.3 Patients with splenomegaly, an unusual finding in AA, should always undergo peripheral blood phenotyping to exclude hairy cell leukemia, which can sometimes mimic the symptoms of AA (i.e., pancytopenia or hypocellular bone marrow).
For treatment purposes, AA is categorically divided into severe and moderate. One definition of severe aplastic anemia (sAA) requires that the individual fulfill two out of three criteria: a platelet count of less than 20,000 cells/μL, a reticulocyte count of less than 60,000 cells/μL or transfusion-dependence, and an absolute neutrophil count of less than 500 cells/μL.4 Patients with neutrophil counts less than 100 cells/μL are said to have very severe AA. Patients with AA who do not fulfill the criteria for severe disease—those with an absolute neutrophil count (ANC) below 1200/μL, a reticulocyte count below 60,000/μL and, platelet count below 80,000/μL—are classified as having moderate aplastic anemia (mAA). The urgency with which therapy is instituted is dictated by the current neutrophil count and the duration of severe neutropenia, which most closely correlates with survival. Although patients with anemia and thrombocytopenia may be supported by red cell and platelet transfusions, very severe neutropenia (ANC < 200/μL) is associated with a high mortality rate and the risk of life-threatening infection. When patients present with higher neutrophil counts, there is more time to explore treatment options.
Pathophysiology
Clinical and laboratory data support the role of the immune system in the pathogenesis of AA. Various immunosuppressive drugs, including antithymocyte globulin (ATG), cyclosporine (CSA), and high doses of cyclophosphamide or corticosteroids, have produced hematologic improvement in the majority of patients with life-threatening cytopenias.5 Responses usually correspond to independence from the need for transfusion and increased neutrophil counts to levels adequate to prevent infection. Laboratory data accumulated over the last two decades have implicated an underlying immune-mediated destruction of blood-forming cells in the pathophysiology of AA. This is manifested in vitro by coculture inhibition of hematopoietic colony formation by patient’s lymphocytes. In vivo, one can detect circulating activated cytotoxic lymphocytes and increased production of cytokines typical of the Th1 response, especially interferon-α (IFN-α), tumor necrosis factor α (TNF-α), and interleukin (IL)-2. Acting locally in the BM, activated T cells likely induce Fas-mediated cell death of hematopoietic progenitor and stem cells, ultimately resulting in a marked decrease in the number of these cells and severe pancytopenia. In support of this hypothesis, we have shown that many patients with sAA have increased expression of CD8 lymphocyte IFN-γ demonstrated by both flow cytometry6 and microarray analysis.7 Examination of T cell receptor variable β (Vβ) subfamilies has shown expansion of specific Vβ subfamilies in many patients with AA suggestive of an antigen-driven immune response; spectratyping shows skewing indicative of oligoclonal expansion within these Vβ subfamilies.8,9
In some patients, genetic abnormalities may place them at risk for AA. Recently, we described mutations in the telomerase TERC gene and the reverse transcriptase itself (TERT gene) in a group of AA patients with short telomeres and markedly reduced telomerase activity.10 Patients’ relatives carrying these mutations also have mild peripheral blood count abnormalities and a normal or reduced stem cell compartment. This disorder may result in progressive telomere shortening leading to early progenitor senescence. Telomerase complex gene mutations may represent a genetic risk factor for bone marrow failure. The fact that some family members with the mutation are asymptomatic and a number of patients respond to immunotherapy, suggests that at least in some cases an additional acquired factor (e.g., immune attack) is required to cause bone marrow failure in some of these patients.
Treatment
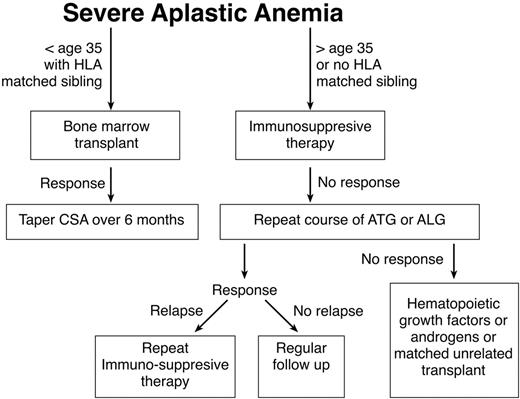
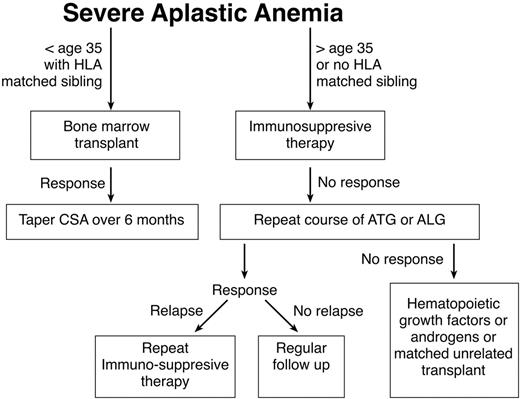
Standard treatment for AA entails immunosuppression or bone marrow transplantation (BMT). Factors such as the patient age, the availability of a matched sibling donor, and risk factors such as active infections or heavy transfusion burden should be considered in determining whether a patient is best treated by transplant or immunosuppression. Any of these factors might lead the patient and his or her physician to choose immunosuppression rather than BMT. Younger patients generally tolerate transplantation better and have fewer long-term problems with graft-versus-host disease (GVHD). In this population the risks of chronic GVHD and transplant-related mortality must be weighed against the high probability of relapse and the risk of developing clonal disease if they are treated with immunosuppression. Older patients and patients with comorbid conditions who may tolerate transplantation less well are generally offered a course of immunosuppression, while younger patients with a matched sibling are advised to pursue transplantation. One restrospective analysis supports this management. Although there were no differences in survival between BMT and immunosuppressive therapy (63% vs 61%) in the overall patient population, significant differences were apparent in favor of BMT for patients younger than 20 (64% vs 38%). Patients older than 20 years with neutrophil counts 0.2–0.5 × 109/L appeared to benefit from immunosuppression in comparison to BMT. In general, patients with severely depressed neutrophil counts tended to do better with BMT, because of the shorter duration of time required for resolution of neutropenia (it should be kept in mind that patients receiving immunosuppressive therapy may respond with improvement of neutropenia as late as 6 months after initiation of therapy). For the middle-aged patient with a matched sibling donor, the recommendation regarding therapy should be made after considering the general health of the patient, the severity of disease, and the desires of the patient. Non-neutropenic patients may be supported for long periods of time without substantial morbidity except for the problem of iron overload. A treatment algorithm seen in Figure 1 may be used as a guide in caring for patients with aplastic anemia.
Immunosuppressive therapy
Most immunosuppressive regimens employ either horse ATG or rabbit antilymphocyte globulin (ALG). The addition of CSA to the treatment regimen results in better response rates.11 A National Institutes of Health (NIH) study5 as well as a European study, combining ATG with CSA resulted in response rates close to 70%. The NIH regimen consists of horse ATG (40 mg/kg per day for 4 days) and 12 mg/kg (adults) and 15 mg/kg in children per day (in divided dose) of CSA for 6 months. Corticosteroids are added (1 mg/kg of prednisone) during the first 2 weeks to ameliorate the serum sickness associated with ATG administration. Addition of G-CSF does not improve the survival rate. Response generally occurs within 6 months and improvement is usually observed between 1 and 2 months. Transfusion independence occurs at 2–3 months, but very late responses are possible. Response rates do not appear to be influenced by the presumed etiology of the disease (e.g., post-hepatitis, toxic exposure, paroxysmal nocturnal hemoglobinuria (PNH)/AA syndrome).
Administration of ATG may be associated with anaphylactic reactions that are not always predicted by a positive skin test. Both ATG and ALG result in rapid reduction in the number of circulating lymphocytes to levels less than 10% of the initial value. Although lymphopenia only persists for several days, the numbers of activated lymphocytes remain decreased for an extended period. Transient depression in platelet and neutrophil counts also can occur. Responses to immunotherapy frequently do not result in completely normal counts but confer transfusion independence or ameliorate the patient’s risk for severe infection due to neutropenia. Some patients who respond to immunotherapy may continue to be dependent on CSA and may develop worsening cytopenias upon discontinuation of the drug. In these patients, the CSA dose should be tapered to the lowest dose possible that maintains acceptable counts. CSA causes renal toxicity and hypertension. Irreversible renal damage may occur as a result of interstitial fibrosis and tubular atrophy, both of which appear to be related to the duration of therapy and the size of the doses received. The efficacy of daclizumab, an IL-2–receptor antibody, is being tested in patients who require continued immunosuppression.
Development of tests predictive of response to immunotherapy would prove useful in helping manage patients unlikely to respond to other therapies. Some studies have proposed predictive tests based on hematopoietic colony formation with and without added T cells, in vitro response of progenitor cells to ALG, IFN-α mRNA measurements in marrow cells, and measurement of early progenitor cell numbers post-ATG treatment. None of these tests adequately distinguishes the 30% of nonresponders. Based on the assumption that most responding patients would show evidence of immune activation prior to treatment, we examined CD8 IFN-α levels in a cohort of sAA patients undergoing therapy with ATG/CSA. We demonstrated by flow cytometry that levels of CD8 intracellular IFN-γ were able to distinguish most responders from nonresponders.6 Ninety-six percent of patients with a population of CD8 cells expressing IFN-γ responded to therapy while 32% of patients not expressing IFN-γ responded. The results need to be validated by further study. Other clinical features discriminating responders from nonresponders have been studied. Patients with very severe AA (neutrophil counts less than 100 cells/μL) receiving immunosuppression have inferior survivals to those with severe AA.12 Patients failing to respond to G-CSF also have an inferior response rate.13 It has been suggested that this subgroup of patients be considered first for BMT if a matched sibling donor is available.
Relapse among responders to immunosuppressive therapy is common. In one European study, the relapse rate was 35% at 14 years,11 but about half of these patients responded to a second course of immunotherapy. In the NIH study many patients required continued immunosuppresion.5 In many cases patients responded after simply increasing the dose of CSA or reinstituting CSA therapy. Because of the high response to a second course of immunosuppression, relapse does not appear to influence survival. An uncontrolled study suggested that high-dose cyclophosphamide produces a higher rate of complete response, free of the late complications such as relapse and clonal evolution.14 In our randomized controlled trial comparing ATG to high-dose cyclosphosphamide, the two drugs did not show statistically different response rates. Cyclosphosphamide treatment also did not eliminate relapse or clonal evoluation15; this treatment also resulted in severe prolonged neutropenia and life-threatening fungal infections in some patients, necessitating termination of the trial.16
Patients with moderate AA have successfully received less aggressive treatment such as androgens and growth factors, but these treatments do not clearly address the immune pathology and may only result in improvement in cytopenias without affecting progenitor cell destruction. Patients may respond to less aggressive immunosuppression. A small study using daclizumab, demonstrated a response rate of around 50% in previously untreated patients (preliminary findings in 17). This antibody against the IL-2R has the advantage of being directed specifically against activated lymphocytes and can be administered as an outpatient. The dose is 1 mg/kg given every 2 weeks for 5 treatments. No significant side effects were seen in patients with moderate AA and responses were durable. Patients not responding to daclizumab may respond to more intensive immunosuppression with ATG.
Treatment of Refractory Severe Aplastic Anemia
Patients not responding to immunosuppressive therapy who have a matched sibling donor and no significant medical problems may be offered BMT. Unfortunately, patients with significant transfusion history may have increased transplant morbidity. Generally, refractory patients without a matched related sibling donor and those who do not opt for transplantation should receive another course of ATG. Some investigators have attempted to increase the degree of immunosuppression, but these efforts have led to increased toxicity without significant improvements in responses. A trial using daclizumab for refractory AA as well as CSA-dependent AA is currently underway at the NIH. Male anabolic hormones may be used as the third-line therapy with response rates as high as 50%–75% in some studies. Some patients do respond to a combination of hematopoietic growth factors, especially when these are chronically administered over prolonged periods of time.
Clonal Evolution
About 10% of patients with AA treated with immunosuppression develop clonal disease in the 7-year period following treatment.5,11 Bone marrow cytogenetic abnormalities can be demonstrated years following successful treatment and may, in fact, be part of the natural history of AA. Androgen-treated patients also have been reported to develop clonal abnormalities. Of 122 patients treated with ATG/CSA at NIH, 13 developed cytogenetic abnormalities (monosomy 7 in 9 patients, 7p deletion in 1, and trisomy 8 in 2). Monosomy 7 generally occurred in those with a minimal clinical response or in those who relapsed with severe pancytopenia. Seven of these patients died, 4 of bone marrow failure and 3 of leukemic progression. Patients with trisomy 8 generally are responsive to immunosuppression but may become dependent on CSA to maintain good counts. In a small study of patients with trisomy 8 in whom fluorescence in situ hybridization (FISH) was performed before and 6 months following immunosuppressive therapy, all 30 patients tested demonstrated increases in the proportion of cells with trisomy 8, though patients with other cytogenetic abnormalities had no change in the proportion of their cytopenias. We have noted years of clinical stability in these patients, some of whom have normal bone marrow histology. In the NIH study, only age was associated with risk for clonal evolution—each decade of life increasing the likelihood of evolution by 40%. Evolution to monosomy 7 correlated with the platelet count at 3 months (3.5-fold increased risk for patients with platelets < 50 × 103/μL).
Although selected retrospective analyses have correlated development of monosomy 7 with sustained administration of G-CSF,18 other studies have failed to demonstrate such association. In the NIH study patients not receiving G-CSF developed monosomy 7. Confounding factors include the high endogenous levels of G-CSF in marrow failure patients as well as the likelihood that the most refractory patients are treated with G-CSF in a non-prospective trial (refractory patients are more likely to develop monosomy 7 even when not treated with G-CSF). Data from ours and other laboratories18 suggest that many of these patients may have had a monosomy 7 clone at disease presentation, not detected by cytogenetics but demonstrable by FISH. In vitro data from our laboratory suggests that the existing monosomy 7 clone may preferentially expand when exposed to pharmacologic doses of G-CSF, while normal bone marrow cells are unaffected.
The reasons for the increased frequency of clonal abnormalities in bone marrow failure are unclear. In a number of these patients, karyotypic abnormalities may be distinguished by FISH at disease presentation. No abnormality in major checkpoint genes has been detected in these patients that might predispose to aneuploidy. Cells with karyotypic abnormalities or the PIG-A mutation may have growth or survival advantages over normal cells in a bone marrow subject to immune attack. With regard to PNH, previous evidence from ours and other laboratories implies a growth advantage for PNH clones relative to “normal” marrow from the same individual; this relative growth advantage may be related to decreased susceptibility to immune attack, as glycosylphosphatidylinositol (GPI)-positive (normal) CD34 cells express apoptotic markers while those of the PNH clone do not. With regard to MDS, we reported that trisomy 8 cells have both a proliferative and survival advantage over normal cells from the same subject, relating to altered gene expression and upregulation of anti-apoptotic proteins. Should monosomy 7 cells be resistant to apoptosis, this may confer a survival advantage. In patients with trisomy 8, we demonstrated T cells with selective cytotoxicity for the abnormal clone. Although the full implications of this finding are unclear, one may envision a scenario where changes in chromosome 8 number are associated with altered antigen expression, leading to immune recognition and later resulting in destruction of normal innocent bystander progenitor cells and bone marrow failure. Certainly much more work needs to be done in order to understand the problem of clonality.
Treatment of Paroxysmal Nocturnal Hemoglobinuria
PNH is clonal disorder resulting from an acquired X-linked somatic mutation of a PIG-A gene in the affected hematopoietic stem cell. Affected cells lack all GPI-linked proteins, including CD55 and CD59 on the membrane of erythrocytes. Absence of these proteins results in a sensitivity to complement-mediated hemolysis. PNH may present as purely hemolytic disease or in association with AA. For patients with AA and PNH, the GPI-negative clone is generally detectable by flow cytometry from first presentation of AA.19,20 In addition to episodes of hemolysis, patients with PNH also are at an increased risk for thrombotic events.
In patients with purely hemolytic PNH, the bone marrow is frequently able to compensate fully for the chronic hemolysis. These patients generally only require folate and sometimes iron supplements. When hemolysis becomes debilitating, steroids have been anecdotally associated with some success, but their chronic usage should be avoided. Therapies directed at limiting complement fixation may soon be tested in a multicenter trial. A humanized anti-C5a monoclonal antibody (mAb) developed for treatment of autoimmune diseases is currently being tested in several clinical trials but has not yet been applied to patients with PNH.
For patients with PNH and AA, treatment is similar to that for AA, where efforts to optimize hematopoiesis are of primary consideration. Most patients with PNH/AA have only a small GPI-negative clone, which is clinically insignificant. For those with significant clones successful immunosuppressive therapy may improve hematopoiesis so that the bone marrow is able to at least partially compensate for ongoing hemolysis.
Patients with PNH have an increased incidence of thrombotic episodes. Risk appears to correlate with the size of the neutrophil PNH clone. A recent study demonstrated the efficacy of anticoagulation with coumadin in these patients, reducing the risk of thrombosis from 36% to 0%.21 Treatment with coumadin may pose a risk to the thrombocytopenic patient and must be balanced against the risk of subsequent thrombosis. The mechanism responsible for thrombosis is unclear. Patients with continued life-threatening thrombotic events should consider BMT, particularly if they have a matched sibling donor. Complete relief of symptomatology has been reported following a successful transplant.
Matched-Related Allogeneic Bone Marrow Transplantation
There has been a steady improvement in the results of BMT in this disorder related to improved conditioning regimens and drugs to control GVHD. Two recent studies using CSA/methotrexate for GVHD prophylaxis estimate 5-year disease-free survivals of 88%22 and 94%.23 Chronic GVHD has been the major factor responsible for the decreased survival rate of adults when compared to children. In one study, 41% of adult patients who survived more than 2 years had chronic GVHD. Their mortality was three times higher than for patients without this complication.
Graft rejection constitutes a greater problem in patients with AA than in those receiving a transplant for other disorders. The increase in the rate of rejection may be related to the immune pathophysiology of AA, as well as to the less intensive conditioning regimens used in this patient population. Most transplant centers reserve total body irradiation for the unrelated BMT, as it has not been shown to confer a survival advantage over cytotoxic therapy alone for the matched related transplant recipient. While intensification of the conditioning regimen results in a lower graft rejection rate, in general it does not affect long-term survival. BMT is associated with several late complications, including solid tumors and MDS. Although most of these late complications are related to total body irradiation (TBI) (e.g., in a French study, solid tumors have developed in the radiation field of 5 of 147 patients), the risk of solid malignancy remains increased in BMT patients who were not treated with TBI. This risk appears to be equivalent in patients treated with immunosuppressive therapy and those treated with BMT (without TBI).
Matched-Unrelated Allogeneic Bone Marrow Transplantation for Aplastic Anemia
Although allogeneic BMT is an option for young patients with a matched sibling donor, only 30% of patients have such a donor. For those young patients not responding to immunosuppression, matched-unrelated allogeneic transplantation may be considered an option. In a large European study, the survival for sibling-matched donors was was 86% compared to 43% for matched unrelated donors.24 For patients with a single mismatch it is 25%, and for 2- or 3-locus mismatches it is 11%. In a recent study from Seattle, addition of TBI to the conditioning regimen increased the survival to 50% in patients receiving a partially matched donor graft.25 Similarly, improved results have been obtained at Children’s Hospital in Milwaukee where pediatric patients receiving a T cell–depleted graft were conditioned with a preparative regimen including cytosine arabinoside, cyclophosphamide and TBI (a survival rate of 54% at 3 years was achieved).26 In general, results of unrelated BMT for adults have been disappointing due to higher rates of graft rejection and GVHD. In patients receiving matched unrelated donor transplants, factors such as age, degree of match, and type of conditioning regimen have a greater impact on survival than in patients receiving a matched sibling donor graft. T cell depletion of the donor graft in combination with a rigorous conditioning regimen including TBI increases the survival and decreases the prevalence of chronic GVHD. However, many adults have difficulty tolerating the conditioning regimen that includes TBI. There is a higher frequency of malignant disease in these patients when compared to those not treated with TBI.
III. The Challenge of Myelodysplasia
Charles A. Schiffer, MD*
Wayne State University School of Medicine, Barbara Ann Karmanos Cancer Institute, 3990 John R. Street, 4HW-CRC, Detroit MI 48201
The myelodysplastic syndromes are a heterogeneous group of disorders characterized by progressive pancytopenia, some of which seems to be the result of increased apoptosis of precursor cells and impairment of cellular differentiation (“ineffective hematopoiesis”) with a tendency to evolve to acute leukemia. The term “syndrome” is indicative of the wide clinical spectrum of MDS, which includes patients with moderate anemia with normal neutrophil and platelet counts, patients with hypocellular or markedly hypercellular bone marrows, and others with frank leukemia; clinical courses can range from a few months to many years. Indeed, it is clear that a number of biologically distinct disorders are combined under this rubric, awaiting better molecular characterization to allow better classification. An overview of current understanding of the biology of MDS, the limitations of current treatments and possible future approaches are available in the proceedings of an National Cancer Institute (NCI)–sponsored “State of the Science” meeting at www.conference-cast.com/webtie/sots/leukemia2/lectures.htm.
MDS may be the most common clonal neoplastic disease of hematopoietic origin in adults, with a suggestion that the incidence is rising, in part because of the aging of the population in the Western world. It is probably underdiagnosed and may be the cause of some of the mild to moderate anemias encountered in older people, often attributed to “anemia of chronic disease.” MDS is a consequence of multiple mutations accumulating over time that affect the hematopoietic stem cell. It is notoriously resistant to chemotherapy with low complete response rates as well as short durations of response. Drug resistance is a feature of virtually all myeloid leukemias deriving from multipotent stem cells, presumably due to further perfection by the neoplastic cells of the multiple mechanisms that protect normal progenitors from damage by exogenous toxins.
In addition, there is often a prolonged period of cytopenia due to delayed recovery of normal hematopoiesis following chemotherapy, a particular problem in the elderly population of patients with MDS. Incomplete marrow recovery can also preclude the delivery of postremission treatment to some responding patients. This is in contrast to patients with de novo acute leukemia, in whom there is a relatively reliable return of normal blood counts following effective cytoreductive chemotherapy. This may suggest a deficiency in either the quality or number of normal stem cells in MDS patients. The mechanism(s) by which the development and proliferation of a dysplastic clone suppresses the growth of residual normal hematopoietic elements is not known. It is also unknown whether recovery of peripheral blood counts following therapy for MDS is attributable to the return of polyclonal hematopoiesis or reflects improved differentiation capacity of the MDS clone.
Recent Standardization of the Definitions of MDS, Response to Treatment and the Influence of Prognostic Factors
Comparisons of different reports of treatment outcome have been hampered by the use of varying definitions of MDS, inclusion of heterogeneous groups of patients with different expected clinical courses, and an absence of agreed upon definitions of response. These issues have been addressed by a number of international committees in recent years. Although their use should produce more consistency in the literature, these efforts have not provided important biological insights or new clues about directions for innovative therapies.
Morphology and classification
A World Health Organization–sponsored committee modified the longstanding French-American-British (FAB) classification of MDS by modifying the definitions of refractory anemia, moving most cases of chronic myelomonocytic leukemia to the myeloproliferative category where they belong, and, with little biologic or clinical rationale, changing the definition of AML from a threshold of 30% to 20% blasts.1 There is some concern that the latter change has resulted in inappropriate recommendations of intensive AML therapy for patients previously classified as MDS (refractory anemia with excess blasts [RAEB] or RAEB in transformation [RAEB-t]) who are unlikely to benefit from such an approach. For example, it is not unusual to see elderly patients with well-characterized prior MDS who are found to have > 20% blasts on a marrow aspirate, in the absence of new clinically significant events or count changes, who have been told that they now have “AML” that must be treated immediately. The factors that influenced outcome with intensive induction when the patient had “MDS” last week are still operative and predict for a poor outcome at this time as well.
In contrast, the 20% definition may be clinically relevant in a recently diagnosed patient with a short or absent cytopenic prodrome, clinical features suggesting a more immediate need for treatment, and the absence of medical conditions precluding intensive chemotherapy. In addition, some younger patients with FAB M2 morphology (sometimes with the favorable translocation t(8;21)) can have fewer than 20% morphologic “blasts” and merit prompt treatment.2
The assessment of hypocellular MDS is complicated by the dearth of cells for qualitative microscopic analysis, although cytogenetic studies can be helpful, particularly in patients with secondary MDS, a condition in which hypocellularity is often present. Notwithstanding these difficulties, the identification of hypoplastic MDS depends upon the application of current standards by which the diagnosis of MDS is made.
Prognosis
The IPSS group has proffered a “staging” system for MDS semiquantifying the not particularly surprising observation that combinations of higher numbers of blasts, complex cytogenetic abnormalities and multilineage cytopenias are associated with more rapid transformation to AML and shorter survival.3 The International Prognostic Scoring System (IPSS) permits comparisons to be made of patient populations in different published studies, can help standardize eligibility criteria for research studies, and can be of assistance in recommendations about the timing of allogeneic stem cell transplantation or other therapies in individual patients. It does not, however, provide new biologic insights or discrete hypotheses to be tested in the clinic or the laboratory.
Definition of response
Transformation to acute leukemia occurs commonly in patients with MDS presenting with higher levels of blasts in the marrow. Nevertheless, the major causes of death are infection and hemorrhage, as well as other medical problems characteristic of an older patient population. Complete responses (CR) are not common after most treatments of MDS and a variety of cumbersome definitions have been used to try to capture the clinically relevant but often “incomplete” improvements in blood counts that are induced by some therapies. Recently, an international group of investigators proposed criteria for CR, partial response (PR) and different degrees of hematologic improvement.4 Although admittedly somewhat arbitrary and subject to future revisions, they should also provide some consistency in reports of treatment outcome. It is also important that published reports distinguish between patients with hematologic improvement that is clinically relevant, such as reduction or elimination of transfusion requirements, as compared to less clinically meaningful changes such as a doubling of the platelet count from a baseline of > 75,000/mm3.
Treatment of Myelodysplasia
Through the years, a wide variety of compounds such as glucocorticosteroids, anabolic steroids, pentoxyllophyline, amifostine, TNFα inhibitors and most recently thalidomide and arsenic trioxide have been used to treat MDS, and hematologic improvement has been demonstrated in a fraction, generally < 20%–30%, of patients with MDS. A few general comments can be offered:
There are very few randomized trials comparing active treatment with the standard of supportive care. Most reports are Phase I/II trials with relatively small numbers of patients with heterogeneous characteristics.
The number of abstracts far exceeds the number of peer-reviewed publications, with drug company symposia and “consulting” meetings becoming an increasing source of information transmission. In particular, “negative” trials are likely underrepresented in the literature.
The hematologic responses seen are rarely complete, occur more frequently in “lower risk” IPSS subgroups, are usually not multilineage, generally are manifested by decreases in RBC transfusion requirements and infrequently last for more than 6 months.
Virtually all therapies are accompanied by systemic side effects, some of which are significant. These include transient or sustained worsening of cytopenias with increased transfusion requirements and/or hospitalization. They also may be associated with appreciable medical costs that may be a major burden, especially for older patients.
Importantly, many of the therapies must be given for many weeks to months before responses are apparent or treatment failure can be declared.
Patient selection is a critical consideration in evaluating response, since most protocols exclude individuals with significant medical comorbidities that are quite common in older patients with MDS. There is no systematic information about the large number of patients who elect not to receive treatment or who are treated on an ad hoc basis off clinical trials.
Thus, the decision about whether to treat individual older patients must balance the potential side effects and the low frequency of response against the current and projected symptoms which the patient is experiencing.
The actions of many of these agents can be quite pleiotropic in vitro and there is little understanding of the mechanisms associated with drug sensitivity and resistance. Of note is that many of these agents have anti-inflammatory properties. In typical hypercellular MDS there is ineffective hematopoiesis with massive intramedullary cell death, associated with increases in the levels of a number of cytokines. It is interesting to speculate whether this intramedullary cytokine “storm” might further restrain hematopoiesis and that suppression of this by nonspecific but potent anti-inflammatory agents might permit some restoration of blood counts in the minority of patients who retain some normal cell differentiation. Thus, the effect may be largely mediated by manipulation of the milieu, rather than a direct effect on the MDS clone.
Selected Specific Treatment Approaches
Demethylating agents: 5-azacytidine and decitabine
There are very few randomized treatment studies, with the exception of a trial comparing 5-azacytidine administered subcutaneously with “best supportive care.”5 The 5-azacytidine group had a reduction in transfusion requirement in approximately one-third of patients as well as a delayed time to leukemic transformation and an improved “quality of life.”6 There was no survival benefit, however, perhaps reflecting the cross-over to 5-azacytidine in nonresponders, and an inexorable rapid falloff in overall survival in both groups. On the basis of these data, 5-azacytidine was recently approved by the US Food and Drug Administration (FDA) for the treatment of all FAB types of MDS. While the long-term results are disappointing (median survival ~19 months in the 99 patients initially randomized to 5-azacytidine), some patients do enjoy substantial clinical benefit. The study also demonstrates the feasibility of conducting randomized trials in MDS, although these studies can be complex and require a minimum of 175–200 patients.
Phase I and II trials have also been published evaluating decitabine,7,8 which is similar in structure and function to 5-azacytidine, but which currently has to be administered intravenously. A randomized Phase III trial, similar in design to the 5-azacytidine study but without a provision for cross-over, has recently been completed. Very preliminary results are available on the company website,9 describing a 10% rate of CR and with a crude survival curve at best reminiscent of the 5-azacytidine results.
Both 5-azacytidine and decitabine are cytotoxic when given in higher doses but have other mechanisms of action, including DNA demethylation. This is particularly true of decitabine when it is administered in lower doses.8 Demethylation potentially reactivates genes that are transcriptionally silenced by DNA methylation of their promoters. The silenced genes are postulated to be critical in terms of providing a proliferation and survival advantage to the malignant cells in some cancers, and restoring their expression can lead to cell death. A great deal remains to be learned about the effect of reactivating specific genes with the additional task of proving whether the induction of previously suppressed gene expression is in fact responsible for any clinical responses observed.10
Thalidomide and CC5013
Since the original description by Raza and colleagues,11 thalidomide has been widely used by many clinicians on an ad hoc basis, although there are few additional series with significant numbers of patients. A report from the North Central Cancer Treatment Group12 described the outcome in 73 patients (43 with lower IPSS scores and 30 with less favorable [1.5–3.5] scores). The starting dose of thalidomide was 200 mg/day with an attempt to increase the dose by 50 mg/week. Only 1 patient achieved a partial response and only 7 had hematologic improvement that was sustained for at least 2 months. Most patients stopped therapy because of side effects or disease progression, and only 44% of patients completed 3 months of treatment. This study can be faulted because of the rapid dose escalation schedule, which may have contributed to the relatively short time on treatment. It is possible that lower doses administered for longer periods of time may have been more effective. Nonetheless, this multicenter study failed to confirm preliminary single institution data and may be representative of the response rates to be expected.
CC5013 (Revlimid, Celgene Corp.) is an orally administered immunomodulatory analog of thalidomide that is appreciably more active than thalidomide in a variety of in vitro systems and that does not have the problems with sedation, constipation and neuropathy that have limited the chronic and higher dose use of thalidomide. List et al13 have reported on a cohort of 45 patients, largely with low IPSS stage disease. Twenty-four of 36 “evaluable” patients enjoyed an erythroid response, including 20 major responses defined as a substantial improvement in hemoglobin or a reduction/elimination of transfusion requirements. The maximum improvement was to a hemoglobin of 12.9 gm/dL with a mean increase of 5.1 gm/dL in responders. Relapses have occurred in 3 of these responders to date. There was striking benefit in the group of patients with the 5q- cytogenetic abnormality, with complete cytogenetic response in 10/11 such patients, only 1 of whom has relapsed thus far. In contrast, 1/7 patients with other cytogenetic abnormalities had a cytogenetic response. The apparent specificity of these cytogenetic responses is different from trials with other agents and is most striking. The mechanism for the apparent disproportionately good effect in the 5q- patients is not known. The major toxicity was myelosuppression, necessitating weekly monitoring of blood counts. Count falls were noted in the 5q– responders, sometimes with slow recovery, perhaps reflecting the impaired residual normal hematopoiesis characteristic of patients with MDS. Two large multi-institutional Phase II trials focusing on patients with lower risk MDS or the 5q– syndrome have recently been completed.
Arsenic trioxide
Arsenic trioxide has been evaluated in small Phase II trials using doses of .25 mg/kg intravenously days 1–5 and 8–12 every 28 days14 or .30 mg/kg daily for 5 days followed by .25 mg/kg twice a week for up to 15 weeks.15 Hematologic responses were noted in 7/28 and 13/50 “evaluable” patients, and were largely erythroid in nature, with only 5 of the 12 “major” responders achieving RBC transfusion independence. Responses were often delayed, occurring after 1.5–5.8 months of therapy, and the treatment was cumbersome because of the need for frequent electrolyte monitoring and electrocardiograms. Grade 3 and 4 myelosuppression, as well as a variety of other side effects, were noted. Further details from full publications are needed to better interpret these data, including information on other patients who were treated but not yet evaluated. It was not obvious whether specific subgroups of patients tended to benefit from treatment and it is presently unclear how or when to utilize arsenic trioxide in patients with MDS or whether these results are superior to what has been noted with other agents, or indeed to supportive care alone, particularly in terms of quality of life.16
Immunosuppressive therapy/hypoplastic MDS
Since the original, perhaps surprising publication from the NIH describing both hematologic improvement and some sustained CRs in a group of MDS patients treated with ATG,17 others have confirmed these findings, although with somewhat lower response rates and concerns about the side effects of this treatment in older patients.18,19 Yazji and colleagues from the MD Anderson Cancer Center reported that 5/31 MDS patients (16%) had varying degrees of hematologic improvement including 1 CR after treatment with ATG and a planned 6 months of cyclosporine.20 The cyclosporine was poorly tolerated in this older patient population and was given for only a median of 1 month. It is unclear whether cyclosporine adds to the results achieved with ATG alone.
Responses are seen most frequently, but not exclusively, in patients with refractory anemia. Elimination of clonal lymphoid populations, which may possibly suppress normal hematopoiesis, has been noted after treatment of MDS with ATG.21 In this regard, both CC5013 and thalidomide are immunomodulatory. Retrospective analyses suggest that responses may be highest in individuals with HLA-DR1522 and further studies to help predict the likelihood of benefit in individual patients are needed. In addition, many patients treated on the original NIH trial had what was felt to be “hypocellular” MDS. It can be quite difficult to distinguish between aplastic anemia and MDS in such circumstances, although the presence of cytogenetic abnormalities, which can develop progressively over time, is more indicative of MDS. It remains unclear whether patients with very hypocellular marrows may benefit preferentially from immunosuppressive approaches. Similarly, there are no data about the response rate following other types of therapy in patients with hypocellular MDS.
Allogeneic Stem Cell Transplantation
Currently, allogeneic stem cell transplantation (SCT) represents the only curative therapy for patients with MDS. Transplantation is usually reserved for younger patients with more advanced disease and is often felt to be precluded in older patients, even in those without other medical problems.23 The use of reduced-intensity conditioning regimens permits transplants in selected older individuals. Ho et al performed reduced-intensity allogeneic transplants in 75 patients with different IPSS prognostic groups of MDS, using largely HLA-identical siblings or unrelated donors (MUD).24 The median age was 54 years (up to age 70) for the sibling transplants and 51 years (up to 65 years) for the MUD recipients. Nontransplant-related mortality was low in both groups (8%) at 200 days. With relatively short follow-up, disease-free survival was 83% in the low-risk INT-1 group patients, 67% in the Int-2 but only 31% in the IPSS high-risk patients. Whether these results are an improvement over what might be seen with supportive care in INT-1 patients requires much longer follow-up and eventually consideration of a comparative trial. Certainly, given the risks of SCT, patients with low-risk disease must be selected very carefully and have some evidence of progressive disease. Higher relapse rates in patients with more advanced leukemias and MDS are an issue following conventional SCT, and it is unknown whether “full” conditioning regimens could have produced better antileukemic effects and fewer relapses. This is an important issue for younger patients with MDS and leukemia to whom either type of transplant could be offered, although at this time it appears to be the informal consensus among transplant physicians to utilize myeloablative conditioning regimens in younger patients for whom this is considered to be an option.
Conclusions
Thus, the list of “20% drugs” expands without an ability to predict responders in advance, although response rates seem to be higher in lower IPSS groups. Given patient demands and the absence of alternative treatments, the decision about off-protocol use of a commercially available agent can be difficult, further complicated by the paucity of complete, peer-reviewed publications documenting its efficacy. Certainly, patients should be referred for exploratory trials if available.
Most trials in MDS have been based on modest preclinical data and have been empiric in nature, with some post hoc rationales offered to explain successes or failures. It is therefore also difficult to design trials of rational combinations of agents. It is hoped that better understanding of the biology(ies) of these heterogeneous disorders will provide more hypothesis-driven approaches in the future. Unfortunately, there is no animal model representative of any of the subtypes of MDS. Gene expression profiles of patients with MDS are beginning to be performed, and carefully designed experiments comparing appropriate subgroups of patients may produce biologically and clinically helpful leads. An important issue in MDS is whether whole cell preparations provide reliable information, and many groups are focusing on CD34+ separated cells in an attempt to study immature precursors closer to the elusive MDS stem cell.
Management algorithm for patients with severe acquired aplastic anemia.