Abstract
This review examines the clinical consequences for the practicing hematologist of remarkable new insights into the pathophysiology of disorders of iron and heme metabolism. The familiar proteins of iron transport and storage—transferrin, transferrin receptor, and ferritin—have recently been joined by a host of newly identified proteins that play critical roles in the molecular management of iron homeostasis. These include the iron-regulatory proteins (IRP-1 and -2), HFE (the product of the HFE gene that is mutated in most patients with hereditary hemochromatosis), the divalent metal transporter (DMT1), transferrin receptor 2, ceruloplasmin, hephaestin, the “Stimulator of Fe Transport” (SFT), frataxin, ferroportin 1 and others. The growing appreciation of the roles of these newly identified proteins has fundamental implications for the clinical understanding and laboratory evaluation of iron metabolism and its alterations with iron deficiency, iron overload, infection, and inflammation.
In Section I, Dr. Brittenham summarizes current concepts of body and cellular iron supply and storage and reviews new means of evaluating the full range of body iron stores including genetic testing for mutations in the HFE gene, measurement of serum ferritin iron, transferrin receptor, reticulocyte hemoglobin content and measurement of tissue iron by computed tomography, magnetic resonance imaging and magnetic susceptometry using superconducting quantum interference device (SQUID) instrumentation.
In Section II, Dr. Weiss discusses the improved understanding of the molecular mechanisms underlying alterations in iron metabolism due to chronic inflammatory disorders. The anemia of chronic disorders remains the most common form of anemia found in hospitalized patients. The network of interactions that link iron metabolism with cellular immune effector functions involving pro- and anti-inflammatory cytokines, acute phase proteins and oxidative stress is described, with an emphasis on the implications for clinical practice.
In Section III, Dr. Brissot and colleagues discuss how the diagnosis and management of hereditary hemochromatosis has changed following the identification of the gene, HFE, that is mutated in most patients with hereditary hemochromatosis, and the subsequent development of a genotypic test. The current understanding of the molecular effects of HFE mutations, the usefulness of genotypic and phenotypic approaches to screening and diagnosis and recommendations for management are summarized.
I. Advances in the Laboratory Evaluation of Iron Deficiency and Iron Overload
Gary M. Brittenham, M.D.*
Department of Pediatrics, Columbia University, College of Physicians & Surgeons, Harkness Pavilion HP550, 630 West 168th Street, New York, NY 10032
Acknowledgments: This work was supported, in part, by research grants from the National Institutes of Health (HL62882, HL57594 and DK49108).
Our understanding of the molecular and cellular mechanisms underlying the absorption, transport, utilization and storage of iron in the body provides the foundation for the use of laboratory means to detect iron deficiency, iron overload and other abnormalities of iron metabolism. Recent rapid progress in studies of iron has led to the characterization of newly identified key proteins, shown in Table 1, that interact with the familiar proteins long known to be participants in iron metabolism—transferrin, transferrin receptor, and ferritin—and greatly refined our appreciation of the intricacies of iron physiology.1 While a variety of new laboratory measures are likely to be developed using these and other still to be recognized proteins or genes of iron metabolism, two are already available for routine clinical use and will be discussed in more detail: (i) measurement of the concentration of the soluble fragment of transferrin receptor in serum in the diagnosis of anemia associated with enhanced erythropoiesis and with tissue iron deficiency, and (ii) detection of mutations in the HFE gene in the diagnosis of the iron overload of hereditary hemochromatosis.
Iron metabolism
The metabolic roles of the proteins in Table 1 are described in detail in reviews1,2,3 or reports4,5,6,7,8 elsewhere and will only be summarized here. The major pathway of internal iron exchange is a unidirectional flow from plasma transferrin to the erythron (defined as the totality of erythroid elements at all stages of development) to the macrophage and back to plasma transferrin.3 About four-fifths of the iron passing through the transferrin compartment each day is flowing to and from the erythron. Physiologically, immature erythroid cells acquire iron from transferrin through a specific transferrin receptor located on the surface membrane. HFE,9 the hemochromatosis gene, produces a protein that binds to the transferrin receptor; this protein may have a role in determining receptor affinity for ferric transferrin but its full role in iron metabolism is still incompletely understood (see below). The iron-transferrin—transferrin receptor-[±HFE] protein complex is internalized within an endosome, which undergoes acidification followed by the release of iron and its transport across the endosomal membrane via the divalent metal transporter 1 (DMT1). Most of the internalized iron is used for hemoglobin synthesis and then enters the circulation in erythrocytes, but small quantities of iron may be stored intracellularly in the iron storage protein ferritin.
Within developing erythroid and other cells, the iron-regulatory proteins IRP-1 and IRP-2 act to control iron availability by translational control of the synthesis of transferrin receptor (increasing iron uptake by the cell) and of ferritin (increasing iron storage). IRPs are cytoplasmic RNA-binding proteins that function in a trans-acting manner on mRNAs that contain an iron-responsive element (IRE), a cis-acting regulatory structure. Functional IREs are found in the 3′ untranslated region of mRNAs for the transferrin receptor, in one of the two isoforms of DMT1, and in the 5′ untranslated region of mRNAs for ferritin, for the erythroid-specific form of δ-aminolevulinic acid synthase, and for mitochondrial aconitase. IRPs function as a nexus connecting intracellular iron availability with cellular iron utilization, erythropoiesis, mitochondrial energy metabolism and cellular responses to inflammation and oxidative stress.10 A homolog of the transferrin receptor, identified as transferrin receptor 2, lacks an IRE; a homozygous nonsense mutation in the gene that encodes transferrin receptor 2 is found in some patients with a form of non-HFE-related hemochromatosis.11
Senescent erythrocytes are phagocytized by specialized macrophages in the spleen, bone marrow, and liver. Within macrophages, the inducible heme oxygenase 14 catabolizes heme, releasing ferrous iron to a Fe-ATPase iron transporter,5 which seems to be responsible for intracellular transmembrane iron transport. The exact means of iron exit from the macrophage is uncertain but may involve ferroportin 1.6,7,8 Ceruloplasmin may be required for the mobilization of iron from macrophages and other tissues and for its oxidation and incorporation into ferric transferrin. Whatever the route and means, macrophages return most of the catabolized erythroid iron to the transferrin compartment, where the cycle recommences. The phagocytosis of aged erythrocytes and flawed immature red cells accounts for almost all of the storage iron found in the macrophages of the liver, bone marrow, and spleen; none or almost none of the storage iron is derived from transferrin. By contrast, the parenchymal cells of the liver may either take iron from or give iron to plasma transferrin.
The remaining one-fifth of iron movement to and from transferrin consists mostly of iron shifted between the plasma and extravascular transferrin compartments, exchanged between extravascular transferrin and parenchymal tissues, or moved to and from hepatocytes. Under certain circumstances, iron may enter cells through transferrin-independent pathways via passive perfusion, fluid-phase endocytosis, or membrane-based transport systems that are still poorly characterized. In some cells, another protein termed SFT for “stimulator of Fe transport” seems to enhance both transferrin and nontransferrin-bound iron transport. Frataxin, a mitochondrial protein expressed in neuronal and cardiac tissue, and ATP-binding cassette 7 (ABC7), a mitochondrial protein expressed in neuronal and erythroid tissue, both seem to be involved in mitochondrial iron homeostasis.
Under normal physiologic conditions, less than 0.05% of the total body iron is acquired or lost each day. DMT1 also serves as the intestinal iron transporter that moves iron from the lumen of the gastrointestinal tract through the apical surface of duodenal enterocytes. Hephaestin is a transmembrane-bound ceruloplasmin homolog that is apparently required for the passage of iron through the intestinal enterocyte. Ferroportin 16,7,8 is also expressed at the basolateral surface of duodenal enterocytes and seems to transport iron into the portal circulation. Ceruloplasmin may also be needed for the mobilization of iron from enterocytes and its oxidation and incorporation into ferric transferrin. This synopsis of the current state of understanding of the proteins involved in iron metabolism is intended to serve as back-ground for the discussions of laboratory assessment of body iron status, the anemia of chronic disease and hereditary hemochromatosis that follow.
Soluble fragment of transferrin receptor
The soluble transferrin receptor is a truncated form (Mr 85,000) of the tissue transferrin receptor that consists of the N-terminal cytoplasmic domain that has probably been proteolytically released from the cell membrane.12 The contribution of the newly recognized transferrin receptor 2 to the plasma pool has not been reported. Commercial immunoassays that can detect the soluble truncated form of the transferrin receptor in human plasma have been approved by the FDA and are now clinically available. The soluble form of the receptor that circulates in the plasma reflects the total body mass of cellular transferrin receptor.13 Because, in normal subjects, over 80% of the mass of cellular transferrin receptor is located in the erythroid marrow, the concentration of circulating soluble transferrin receptor is primarily determined by erythroid marrow activity. Accordingly, decreased levels of circulating soluble transferrin receptor are found in patients with erythroid hypoplasia (aplastic or hypoplastic anemia, chronic renal failure). Increased levels are present in patients with erythroid hyperplasia (thalassemia major, sickle cell anemia, chronic hemolytic anemia). Iron deficiency is the other principal cause of elevated concentrations of soluble transferrin receptor because, as noted above, with intracellular lack of iron, the iron regulatory proteins direct increased synthesis of transferrin receptor, which is eventually shed into the plasma. In the absence of other conditions causing erythroid hyperplasia, an increase in concentration of plasma transferrin receptor provides a sensitive, quantitative measure of tissue iron deficiency. Most importantly, the plasma transferrin receptor concentration is not increased with infection or inflammation, unlike plasma ferritin. As a result, measurement of the plasma transferrin receptor concentration may be especially helpful in the task of differentiating between the anemia of iron deficiency and the anemia associated with chronic inflammatory disorders, the “anemia of chronic disease.”12 ,14,15,16 The most sensitive means available to distinguish between the anemia of iron deficiency and the anemia of chronic disease is a combination of plasma transferrin receptor and plasma ferritin concentrations, the “transferrin receptor-ferritin index,” i.e. the transferrin receptor concentration divided by the plasma ferritin concentration (or, in some studies, by the log of the plasma ferritin concentration).12 ,14,15,16
Mutations in HFE in Patients with Hereditary Hemochromatosis
First identified in 1996, HFE, the protein that is defective in most patients with hereditary hemochromatosis, is structurally similar to major histocompatibility complex (MHC) class I proteins.9 HFE is a single polypeptide with a short cytoplasmic tail, a membrane-spanning region, and three extracellular domains. The exact role of HFE in iron metabolism and the means whereby mutations result in the increased iron absorption found in hereditary hemochromatosis are still unknown, but a number of potentially relevant observations have been made.3 In the endoplasmic reticulum, newly synthesized HFE forms a 1:1 complex with β2-microglobulin, and the HFE/β2-microglobulin heterodimer is targeted to the plasma membrane. At neutral pH, the HFE/β2-microglobulin heterodimers form a stable complex with the transferrin receptor and apparently decrease the affinity of the transferrin receptor for transferrin. HFE is abundantly expressed in the crypt cells of the duodenal mucosa, and some evidence suggests that levels of HFE and DMT1 may be reciprocally related in intestinal cells. Missense mutations in the HFE gene are responsible for about 85% of cases of hereditary hemochromatosis in the United States15 ; elsewhere the proportion ranges from about 60 to 100%. The most prevalent (homozygous in 83% of patients in the US) is a C282Y mutation. A second mutation in HFE, H63D, was enriched in patients who were compound heterozygotes for the C282Y substitution. Recently, a third relatively common mutation, S65C, has been identified17 and several other isolated or rare mutations have been reported.18,19 Importantly, in the US 10 to 15% of the patients examined have none of the three mutations (C282Y, H63D, S65C) but are indistinguishable clinically from the others.
II. Advances in the Diagnosis and Management of the Anemia of Chronic Disease
Günter Weiss, M.D.*
Department of Internal Medicine, University Hospital Innsbruck, Anichstrsse 35, A-6020 Innsbruck, Austria
Acknowledgments: Support by the Austrian Research Funds FWF 14215 is gratefully acknowledged.
The anemia of chronic disease is the most common cause of anemia in hospitalized patients. This form of anemia typically develops in patients suffering from chronic inflammatory disorders that involve activation of cellular immunity, such as patients with chronic infections, autoimmune diseases or neoplasia. The anemia of chronic disease can usually be easily diagnosed although the underlying mechanisms are not fully understood. A variety of processes have been shown to be involved in the pathogenesis of the anemia of chronic disease, including a diversion of iron traffic from the serum to stores within the reticuloendothelial system, diminished erythropoiesis, a blunted response to erythropoietin, and possibly erythrophagocytosis and a decreased red cell survival. Overall, the anemia of chronic disease seems to be the product of an activated immune system using a defensive strategy of withholding iron, an essential growth factor, from invading pathogens while increasing the efficacy of cell-mediated immunity.
Diagnosis of the Anemia of Chronic Disease
The anemia of chronic disease is generally a normochromic or slightly microcytic anemia, which can be readily diagnosed by laboratory studies of iron status. Patients with the anemia of chronic disease are clinically characterized by reduced plasma iron concentrations and transferrin saturation, while iron stores, as reflected by plasma ferritin levels, are normal or even increased. Transferrin concentrations are at the lower limit of normal or reduced, and reticulocyte counts are usually decreased despite the presence of anemia.1,2,3,4 As noted in the preceding section, the differential diagnosis between iron deficiency anemia and the anemia of chronic disease can now be readily made by measurement of the plasma transferrin receptor concentration and, ideally, determination of the plasma transferrin receptor-ferritin index (i.e. the transferrin receptor concentration divided by the plasma ferritin concentration or, in some studies, by the log of the plasma ferritin concentration). Characteristic findings of laboratory measures of iron status in the anemia of chronic disease and in iron deficiency anemia are shown in Table 2.
Pathophysiology of the Anemia of Chronic Disease
A variety of mechanisms have been identified that contribute to the pathogenesis of the anemia of chronic disease.
Diversion of iron traffic from erythropoiesis to storage sites
The induction of hypoferremia by increased iron retention and storage within the reticuloendothelial system, thereby limiting iron availability to the erythron, is a central feature of the development of the anemia of chronic disease. These alterations in iron metabolism are primarily produced by cytokines and their metabolic products. Almost twenty years ago, treatment of mice with the proinflammatory (Th-1-derived) cytokines IL-1 and TNF-α was shown to induce both hypoferremia and anemia.5,6 These cytokines stimulate ferritin synthesis in macrophages and in hepatocytes via both transcriptional and translational pathways.7,8 Interestingly, not only pro-inflammatory but also anti-inflammatory cytokines play a major role in iron regulation in inflammatory conditions. In activated murine macrophages, IL-4, IL-10 and IL-13 modulate iron metabolism by two different pathways9 : first, by opposing IFN-γ-mediated activation of IRP, thereby increasing ferritin translation, and second, by augmentating transferrin receptor mRNA expression, most likely by reversing the inhibitory effect of IFN-γ on transferrin receptor transcription. Thus, anti-inflammatory (Th-2-derived) cytokines are able to increase iron retention in activated macrophages and may also contribute to the development of anemia.
Apparently, both pro- and anti-inflammatory cytokines participate in the induction of hypoferremia and hyperferritinemia in chronic inflammatory disorders. Part of the action of these cytokines is indirect, via the activation or deactivation of processes involved in the generation of nitric oxide, hydrogen peroxide or superoxide anion. The latter substances affect iron homeostasis via their influence on posttranscriptional regulation of ferritin and transferrin receptor expression by modulating the avidity of iron-regulatory proteins for iron-regulatory elements (IREs) within the untranslated region of ferritin mRNA and transferrin receptor mRNA.
Inhibition of erythroid progenitor proliferation and differentiation
Apart from modulating iron homeostasis, cytokines directly affect erythropoiesis by inhibiting the growth of erythroid progenitors. As we know from the work of Means and Krantz3 and others a number of cytokines, such as TNF-α, IFN-γ and Type I interferons, block BFU-E and CFU-E colony formation. IFN-γ appears to be the most potent inhibitor of erythropoiesis in directly blocking CFU-E proliferation,10 probably accounting for the inverse correlation of IFN-γ levels with hemoglobin concentrations and reticulocyte counts.11 The inhibitory effects of TNF-α or IFN-γ on erythropoiesis may also be related to their ability to induce the formation of nitric oxide. Nitric oxide can directly block erythropoiesis by retarding the proliferation of erythroid progenitor cells in the bone marrow,12 an action mediated, in part, by inhibitory effects of nitric oxide on heme biosynthesis.
Blunted erythropoietin response
Plasma erythropoietin concentrations in patients with the anemia of chronic disease are normal or even increased when compared to healthy subjects. While some studies have suggested that erythropoietin levels are nonetheless low when the degree of anemia is taken into account,13 others (e.g. in juvenile chronic arthritis) have found no significant differences.14 These results suggest that erythropoietin concentrations may vary with the disorder responsible for the anemia of chronic disease. Moreover, erythropoietin responsiveness may also be related to the severity of disease and the amounts of circulating cytokines. Data in vitro have shown that greater amounts of erythropoietin are needed to restore CFU-E colony formation with high concentrations of IFN-γ or TNF-α.15
Erythrocyte survival
In mice, administration of sublethal doses of TNF-α or endotoxin not only decreased the incorporation of iron into red blood cells and induced hypoferremia but also decreased red cell survival.16 In humans, the extent to which a decrease in red cell survival contributes to the anemia of chronic disease is uncertain. Erythrophagocytosis by macrophages could potentially contribute to the destruction of circulating red blood cells and could help explain the development of splenomegaly and the observations of increased amounts of erythrocyte-derived iron in splenic macrophages and Kupffer cells in inflammatory conditions,17,18 but definitive evidence of increased red blood cell destruction in the anemia of chronic disease is lacking.
Advantages of the Anemia of Chronic Disease
The high prevalence of the anemia of chronic disease suggests that the development of this form of anemia might have some benefits for those with chronic inflammation. Two major advantages may be postulated. First, iron is an essential element for all living and proliferating organisms, being required for enzymes in the citric acid cycle, for mitochondrial respiration, for DNA synthesis and for oxygen transport systems. Thus, withdrawal of iron by increased storage of the metal within the reticuloendothelial system acts to limit the availability of iron to microorganisms or tumor cells and thereby inhibit their growth and proliferation. In addition, decreased formation of hemoglobin by withholding iron from the erythron and by cytokine-mediated inhibition of erythropoiesis reduces the oxygen transport capacity of the blood and decreases the overall oxygen supply, which may primarily affect rapid proliferating (malignant) tissues and micro-organisms. Second, iron strongly affects cell-mediated immune function. In addition to the role of iron in the proliferation and differentiation of lymphocyte subsets,19 iron directly inhibits the activity of IFN-γ.20 This crucial pro-inflammatory cytokine is centrally involved in the co-ordination of cell-mediated immune effector mechanisms against invading pathogens. Iron-loaded macrophages exhibit diminished IFN-γ responsiveness, decreased TNF-α production, reduced formation of nitric oxide,20,21,22 and an impaired immune defense against various intracellular pathogens and viruses.23 Therefore, withdrawal of metabolically active iron from the circulation and storage of the metal, as occurs in the anemia of chronic disease, may act to strengthen the immune response via stimulation of IFN-γ-mediated immune effector mechanisms.24
Therapy for the Anemia of Chronic Disease
The optimal treatment for the anemia of chronic disease is cure of the underlying disorder. Even when cure is not possible, immunological therapies such as the use of anti-TNF-antibodies may improve the anemia of chronic disease by counteracting the detrimental effects of TNF-α on erythropoiesis and iron metabolism.
For patients with the anemia of chronic disease associated with chronic infection or malignancy, supplementation of iron should be strictly avoided. First, supplementation of iron may counteract the iron-withholding strategy of the body and favor the growth and proliferation of microbes and tumor cells. Second, iron therapy may weaken cell-mediated immune effector mechanisms and promote progression of the underlying disease.4 Third, most of the administered iron will be diverted to the reticuloendothelial system and little is likely to reach the erythron.
In contrast, iron supplementation could conceivably benefit patients with the anemia of chronic disease associated with auto-immune or rheumatic disorders. In this setting, an iron-induced weakening of cell-mediated immunity might help to reduce disease activity and improve the anemia of chronic disease by counteracting TNF-α or IFN-γ activity, thereby improving erythroid progenitor proliferation, improving endogenous erythropoietin formation and responsiveness, and enhancing the delivery of iron to the erythron by amelioration of disturbances of iron metabolism. Conversely, some investigators have evaluated the use of iron chelation with deferoxamine for treatment of the anemia of chronic disease in rheumatic disorders, despite a low serum iron concentration. In one report, iron chelation therapy was associated with a rise in hemoglobin levels, which was attributed to stimulation of erythopoietin production25 via induction of hypoxia regulatory factors in the erythopoietin promoter.
Blood transfusion has been used to correct the anemia of chronic disease, especially in cancer patients where other mechanisms (e.g. bone marrow suppression following chemotherapy, bone marrow infiltration) may contribute to the anemia. Recombinant erythropoietin may also be used for the management of the anemia of chronic disease,26 but response rates vary and are often low.27 We need to better define therapeutic endpoints and aims of treatment for the use of recombinant erythropoietin in the anemia of chronic disease. We also need to develop new models for choosing those patients most likely to benefit from recombinant erythropoietin and for predicting the likelihood for a response to treatment; these might include not only measures of iron homeostasis but also soluble markers reflecting activated cell-mediated immune function in vivo, such as β2-microglobulin, neopterin or IFN-γ.28,29
III. Advances in the Diagnosis and Management of Hereditary Hemochromatosis
Pierre Brissot, M.D.,* Fabrice Lainé, Anne Guillygomarc'h, Dominique Guyader, Romain Moirand, and Yves Deugnier
Clinique des Maladies du Foie and Liver Research Unit, INSERM U-522, University Hospital Pontchaillou, 35033 Rennes, France
Hereditary hemochromatosis1 is one of the most frequent genetic diseases in Caucasian populations, affecting approximately one in three hundred people.2 The discovery, in 1996,3 of the HFE gene that is mutated in most patients with hereditary hemochromatosis has rapidly provided a powerful diagnostic genotypic test. The availability of this genetic test has radically transformed the diagnostic strategy and overall management of the disease.
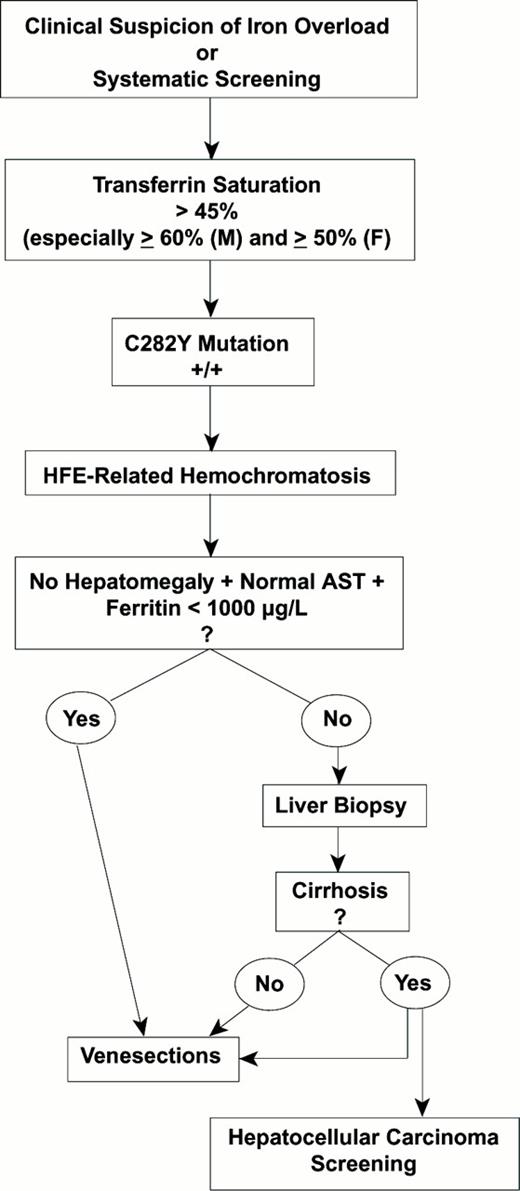
Strategy for the Diagnosis of Hereditary Hemochromatosis
Strategy for the diagnosis of hereditary hemochromatosis.
Abbreviations: M, male; F, female; AST, aspartate aminotransferase
Strategy for the diagnosis of hereditary hemochromatosis.
Abbreviations: M, male; F, female; AST, aspartate aminotransferase
First Step: Suspect the Diagnosis from Early Clinical Manifestations
Suspecting hemochromatosis is easy with the “classical” clinical picture of the disease: A middle-age man presenting with i) diffuse hyperpigmentation (melanodermia), often with a metallic grey or “bronze” rather than a brown discoloration; ii) hepatomegaly, with the liver markedly enlarged, firm and sharp to palpation but without signs of hepatocellular insufficiency (no palmar erythema, no spider nevi, no bruises, normal prothrombin time) or of portal hypertension; and iii) diabetes mellitus, often requiring insulin. When this classical triad of “bronzed cirrhosis with diabetes” is present, the diagnosis may be immediately suspected, especially if there are also signs of cardiomyopathy. This presentation occurs only when complications are irreversible and the prognosis is already poor, so that making the diagnosis at this stage is far too late and must be considered a diagnostic failure.
Earlier signs and symptoms that may allow an earlier diagnosis include:
Sex and age; women can be as severely affected as men5 and both young adults and older women are at risk.6
Three types of earlier signs, which can be summarized as the “rule of three A's”: (i) Asthenia: unexplained chronic fatigue, sometimes with a sexual component in males, can be the only presenting feature of the disease. Paradoxically, iron overload is sometimes discovered when iron deficiency is suspected. (ii) Arthralgia: arthropathy is a frequently misdiagnosed presenting feature of hereditary hemochromatosis, often with a diagnostic delay of 4 to 10 years. The most characteristic expression is chronic arthritis of the second and third metacarpophalangeal joints, resulting in a “painful hand-shake,” a symptom which should be highly suggestive of the disease. Other joints can also be affected, especially wrists and knees. Patients can also suffer attacks of pseudo-gout (pyrophosphate arthropathy). Radiologically, the most common changes are subchondral arthropathy and chondrocalcinosis. Arthropathy greatly diminishes the quality of life in hemochromatosis. (iii) Aminotransferase (transaminase) elevation: any hypertransaminasemia, less than three times the upper normal limit of normal and unrelated to alcohol, non-alcoholic steato-hepatitis (NASH), viral infection, auto-immunity or drugs may reflect hepatic iron overload.
Other possible, but non-specific, features of hereditary hemochromatosis include ichthyosis and nail abnormalities, especially on the first three digits, such as platonychia or true koilonychia, the latter sign being rather paradoxical since this finding may also be also present with chronic iron deficiency.
Second Step: Screen for Biochemical Abnormalities of Iron Metabolism with the Serum Transferrin Saturation
Normal transferrin saturation (< 45%) rules out the iron overload of hereditary hemochromatosis in the absence of a coexisting inflammatory syndrome (as reflected, for example, by an increased serum C-reactive protein [CRP]). Nonetheless, a normal transferrin saturation is compatible with the two types of iron overload not related to hereditary hemochromatosis:
The recently described insulin resistance-associated liver siderosis,7 also termed “dysmetabolic hepatosiderosis,” a condition with mild or moderate iron excess occurring especially in men with features of insulin resistance (increased body mass index, diabetes, hyperlipidemia). The transferrin saturation is normal but the serum ferritin is elevated.8 This disorder is often mistaken for hereditary hemochromatosis and is still poorly understood.
Hereditary aceruloplasminemia,9 a rare disorder due to a mutation in the ceruloplasmin gene located on chromosome 3. Aceruloplasminemia mimics hereditary hemochromatosis in being familial and may be associated with marked hepatic iron overload and diabetes mellitus. Despite these similarities, in addition to the decreased transferrin saturation, two major features suggest the diagnosis of aceruloplasminemia: a) the combination of marked hyperferritinemia with a low transferrin saturation (and, in some cases, anemia) in the absence of an inflammatory syndrome; and b) the presence of neurological abnormalities (extrapyramidal syndrome, cerebellar ataxia, dementia, etc.), which are never present in hereditary hemochromatosis. The diagnosis is established by an undetectable serum ceruloplasmin concentration.
An increased transferrin saturation reflects the basic metabolic abnormality of hereditary hemochromatosis and is the most sensitive single test for phenotypic detection of the disease. Edwards and Kushner10 have shown that the transferrin saturation is usually above 60% in men and 50% in women and remains high throughout the day11 in these patients. Although a sensitive marker, an elevated transferrin saturation is not specific for hereditary hemochromatosis. An increased transferrin saturation can be found in other iron overload syndromes of hematological origin where the major mechanisms responsible for iron excess are dyserythropoiesis, with or without red blood cell transfusions. With these conditions, the key differential feature with respect to hereditary hemochromatosis is the presence of chronic anemia, although the anemia may be very mild. The transferrin saturation can also be elevated in the absence of any iron excess in case of hepatic cytolysis (as indicated by increased levels of serum transaminases) especially when associated with hepatic failure (resulting in decreased transferrin synthesis) and with excessive alcohol consumption.
Third Step: Prove the Diagnosis of Hereditary Hemochromatosis
Confirmation of the diagnosis may be obtained with a single blood test for the HFE mutation C282Y. Three situations occur:
The patient is C282Y +/+ (homozygous for this mutation). The genotypic diagnosis of hereditary hemochromatosis is established and no further investigations are needed to confirm the diagnosis. At this point, evaluation of the degree of iron overload and of the possible visceral or metabolic consequences of the disease is needed.
For evaluating iron excess, two main investigations are valuable: (1) One is biochemical and widely accessible: the concentration of serum ferritin, which, in hereditary hemochromatosis, is generally in good correlation with the total iron burden, provided certain precautions are taken in interpretation: (a) Overestimation of the magnitude of the iron overload may result from confounding factors such as inflammation, cytolysis or a dysmetabolic syndrome. (b) Underestimation of the magnitude of the iron overload may result if the data are interpreted only by reference to the normal upper limits of the serum ferritin. (2) The other investigation, still under study, is the use of non-invasive techniques for the evaluation of hepatic iron, such as hepatic magnetic resonance imaging (MRI)13 or Superconducting Quantum Interference Device (SQUID) susceptometry.
For evaluating the visceral and/or metabolic consequences of hereditary hemochromatosis, a general workup should include serum transaminases, glucose studies and, depending on the clinical context, electro-/echocardiogram, joint and bone x-rays, and hormonal tests. A major difficulty for the clinician is to decide whether a liver biopsy should be performed to detect the possible development of severe fibrosis. This decision constitutes a real dilemna because liver biopsy remains an invasive procedure that should not be performed if the risk of fibrosis is only minimal but, on the other hand, it is essential not to miss marked hepatic fibrosis because of the high risk for subsequent development of hepatocellular carcinoma. Guyader et al14 have recently helped to define the criteria that, in practice, allow a C282Y +/+ patient to avoid a liver biopsy because of the minimal risk of hepatic fibrosis. These criteria are the absence of hepatomegaly and normal serum aspartate aminotransferase and serum ferritin < 1000 μg/L.
The patient is C282Y +/- (heterozygous for this mutation). The most likely genetic status is that of a compound heterozygote that corresponds to various genotypic situations. The most common genotypic profile is a C282Y/H63D compound heterozygote (the patient is C282Y+/- and H63D +/-). In this setting, iron overload remains generally mild so that possible associated cofactors of iron overload must always be searched for (such as excessive alcohol consumption, dysmetabolic hepatosiderosis or porphyria cutanea tarda).15 More rarely, another type of compound heterozygote (i.e. not involving H63D) may be present, most of which are still being clinically investigated, e.g. IVS3 + 1G→T,16 which has been reported in association with severe phenotypic expression of hemochromatosis, the S65C mutation17 associated with a mild phenotype, and the I105T mutation.18
In practice, when iron overload is found in a heterozygote for the C282Y mutation, a search for the H63D mutation is reasonable, but even if a compound heterozygote is found, possible co-factors for iron overload should still be sought. In most cases, simple heterozygotes for C282Y (i.e. C282Y +/- and H63D -/-) do not develop iron overload, and other contributing factors need to be found.
The patient, despite a phenotypic picture of hemochromatosis, is C282Y -/-. Two possibilities should then be considered: i) Juvenile hemochromatosis. This rare disease is now recognised as being genetically independent of hemochromatosis because the causal mutation is located on chromosome 1.19 The diagnosis should be suspected in subjects less than 30 years of age with heart failure, endocrine disorders (hypogonadotropic hypogonadism) or both. ii) Inherited non-HFE-related hemochromatosis is an entity that has been described in most detail in Italian families20 ; some cases of non-HFE-related hemochromatosis have now been found to be homozygous for mutations in the gene for transferrin receptor 2.21
In practice, whenever there is a strong suspicion of pronounced iron overload and the patient is not C282Y +/+, one must remain a “clinician” and resort to a liver biopsy for a diagnostic purpose, accordingly to the “pre-HFE” diagnostic strategy. Indeed, in this situation, hepatic histology is essential in many diagnostic aspects: i) to confirm iron overload; ii) to identify a predominantly periportal and hepatocytic distribution; iii) to provide a semi-quantitative evaluation of iron excess using a special grading system22 ; iv) to permit the determination of hepatic iron concentration (HIC), which is closely correlated with the level of iron stores23 and can be performed in deparaffinized liver biopsy specimens.24 Furthermore, given the age of the patient, the hepatic iron index (ratio of hepatic iron concentration to age) can be calculated. Prior to the HFE era, a value of the hepatic iron index greater than 1.9 was highly suggestive of homozygous hemochromatosis,25 provided other kinds of iron overload (especially of hematological origin) had been excluded. Finally, v) liver biopsy is able to detect associated lesions (e.g. steatosis).
Management of Hereditary Hemochromatosis
Two aspects of the management of hereditary hemochromatosis will be considered: curative management and preventive management.26
Curative management
Apart from the symptomatic treatment of visceral and metabolic complications for the disease, which will not be reviewed here due to their lack of specificity, the major curative challenge is the elimination of iron excess.
Venesection therapy is the key tool. The removal of one unit of blood per week, resulting in the loss of 200-250 mg of iron, should be conducted until the serum ferritin is ≤ 50 μg/L and the transferrin saturation is ≤ 20%, provided hemoglobin levels do not drop below 110 g/L. Thereafter, maintenance phlebotomies must be performed throughout the patient's life to keep serum ferritin ≤ 50 μg/L and transferrin saturation ≤ 35% (a level that usually corresponds to the disappearance of non-transferrin bound iron,27 a potentially toxic iron species28 ). The efficacy of venesections is excellent. The life expectancy returns to normal, provided neither cirrhosis nor diabetes were present at the time of the diagnosis.29 Even with cirrhosis, the prognosis is far better than for other types of cirrhosis, especially of alcoholic origin. With regard to the various syndromes of the disease, the efficacy of phlebotomies is variable: i) Good for asthenia, skin pigmentation, and hypertransaminasemia; ii) Inconstant for arthralgia (which may even worsen during—and sometimes after—the iron depletion treatment), for glucose abnormalities, and for non-cirrhotic fibrosis (which can stablize or decrease); iii) Poor for impotence; iv) Ineffective for two types of lesions: a) cirrhosis, which is an irreversible process, and b) hepatocellular carcinoma, which may develop in cirrhotic patients despite adequate iron elimination by phlebotomies.
Dietary recommendations are useful for counteracting iron excess: alcoholic beverages should be avoided, supplemental iron and supplemental vitamin C are contra-indicated, and tea is beneficial.
In the following cases, venesection is contra-indicated:
Cases of mild iron overload. A 400-500 mL weekly regimen of blood removal may be unnecessary or imperfectly tolerated. A pragmatic attitude is to test the tolerance of the patient for phlebotomies, for instance, beginning with 200-300 ml per week and progressively increasing the volume, possibly up to the “standard” schedule.
Clinically asymptomatic and young individuals. It seems reasonable to begin venesection therapy only when patients are at least 18 years of age, considering that iron needs are important during infancy and adolescence, and that in two large series of asymptomatic patients29,30 the youngest subjects were 18 and 19 years old.
Preventive management
Preventive management of hereditary hemochromatosis has greatly evolved since the discovery of the HFE gene.
Family Screening: The preventive strategy has been considerably modified and simplified by HFE testing. Starting from a C282Y proband, it is now possible to evaluate “immediately” the hemochromatosis risk among the family members. In brief, C282Y +/+ subjects are homozygous for the HFE gene and either already expressing the disease or are at high risk of developing it. C282Y +/- individuals are heterozygous for the HFE gene. They will not develop the disease but can transmit the gene to their offspring. Due to the high prevalence of the HFE gene in the general population, the probability for a heterozygote to marry another heterozygote is approximately 10%. It is therefore important to inform the family of this possibility in a “smooth and positive” way. With respect to young family members, specific screening is not justified because no treatment is indicated during infancy and adolescence. A phenotypic evaluation (including clinical examination, serum transferrin saturation and ferritin) can be performed at 15 years of age and genetic testing postponed until 18 years of age.
Population screening: Several arguments can be put forward in favor of general screening in Caucasian populations: i) the high frequency of the disease; ii) the severity of the disease both in terms of morbidity and mortality; iii) the possibility, from now on, of establishing the diagnosis on the basis of non-invasive investigations; and iv) the efficacy and simplicity of the treatment (venesections), which not only improves the quality of life but restores normal life expectancy provided the diagnosis is sufficiently early in the course of the disease.
The screening strategy could be based on the assessment of serum transferrin saturation in adults aged 18 or more. Genetic testing testing for C282Y would be confined to individuals with transferrin saturation > 45%. This strategy would then avoid the ethical, logistical, and financial problems raised by systematic genetic testing as well as the societal impact of discovering a genetic mutation in asymptomatic persons without a disease. It is, in fact, essential that major changes occur in the attitudes towards unexpressed or slightly expressed HFE homozygosity, especially by insurers and health care administrators, to avoid any adverse genetic discrimination.
Summary
In conclusion, hemochromatosis is a striking illustration of a disease in which immediate clinical benefit has been obtained from a basic discovery at the molecular level. It is indeed a paradoxical disease, connecting a treatment worthy of the middle ages with a diagnostic procedure of the 21st century.