Abstract
This review addresses three related bone marrow failure diseases, the study of which has generated important insights in hematopoiesis, red cell biology, and immune-mediated blood cell injury. In Section I, Dr. Young summarizes the current knowledge of acquired aplastic anemia. In most patients, an autoimmune mechanism has been inferred from positive responses to nontransplant therapies and laboratory data. Cytotoxic T cell attack, with production of type I cytokines, leads to hematopoietic stem cell destruction and ultimately pancytopenia; this underlying mechanism is similar to other human disorders of lymphocyte-mediated, tissue-specific organ destruction (diabetes, multiple sclerosis, uveitis, colitis, etc.). The antigen that incites disease is unknown in aplastic anemia as in other autoimmune diseases; post-hepatitis aplasia is an obvious target for virus discovery. Aplastic anemia can be effectively treated by either stem cell transplantation or immunosuppression. Results of recent trials with antilymphocyte globulins and high dose cyclophosphamide are reviewed.
Dr. Abkowitz discusses the diagnosis and clinical approach to patients with acquired pure red cell aplasia, both secondary and idiopathic, in Section II. The pathophysiology of various PRCA syndromes including immunologic inhibition of red cell differentiation, viral infection (especially human parvovirus B19), and myelodysplasia are discussed. An animal model of PRCA (secondary to infection with feline leukemia virus [FeLV], subgroup C) is presented. Understanding the mechanisms by which erythropoiesis is impaired provides for insights into the process of normal red cell differentiation, as well as a rational strategy for patient management.
Among the acquired cytopenias paroxysmal nocturnal hemoglobinuria (PNH) is relatively rare; however, it can pose formidable management problems. Since its first recognition as a disease, PNH has been correctly classified as a hemolytic anemia; however, the frequent co-existence of other cytopenias has hinted strongly at a more complex pathogenesis. In Section III, Dr. Luzzatto examines recent progress in this area, with special emphasis on the somatic mutations in the PIG-A gene and resulting phenotypes. Animal models of PNH and the association of PNH with bone marrow failure are also reviewed. Expansion of PNH clones must reflect somatic cell selection, probably as part of an autoimmune process. Outstanding issues in treatment are illustrated through clinical cases of PNH. Biologic inferences from PNH may be relevant to our understanding of more common marrow failure syndromes like myelodysplasia.
I. Acquired Aplastic Anemia
Neal S. Young, M.D.*
National Institutes of Health, Building 10, Room 7C103, Bethesda MD 20892-1652
Aplastic anemia, the paradigm of bone marrow failure syndromes, is defined as pancytopenia and an empty bone marrow. Although not a common disease, aplastic anemia has a social impact disproportionate to its incidence. The presentation of the patient can be dramatic—often a pallid, seemingly bruised young person with frighteningly low blood counts. The striking marrow pathology has invited speculation and later experimentation to determine a pathophysiology responsible for the complete disappearance of the hematopoietic organ. Early association of aplasia with benzene exposure led to heroic industrial hygiene efforts to protect workers from toxicity and ultimately to the virtual disappearance of benzene as a causative factor in hematologic diseases in the United States. An epidemic of aplastic anemia appeared to follow the introduction of chloramphenicol in the 1960s, and the disease has been linked by case reports and formal epidemiologic studies to many classes of pharmaceuticals widely used in medical practice. Because aplastic anemia is such a feared toxicity, even a few cases can have a profound impact on new drug development and on the pharmaceutical industry, as most recently illustrated by the fate of the antiepileptic felbamate. Epidemiologically, aplastic anemia has a pattern of geographic variation opposite to the leukemias, with higher frequency in the developing world than in the industralized West. Finally, and most gratifyingly, the success of treatments, both stem cell transplantation and immunosuppressive regimens, invite application to related hematologic syndromes and indeed to other medical diseases that share its underlying pathophysiology.
Clinical Features
Blood counts determine the presentation and the prognosis. Symptoms of anemia and mucocutaneous hemorrhage usually prompt medical attention. Prognosis is directly related to the reduction in peripheral blood counts, particularly the neutrophil number: < 200 granulocytes/μL defines the category of super-severe disease. In the early twentieth century, patients often died within days or weeks of congestive heart failure, profuse hemorrhage, or overwhelming infection; recurrent bacterial sepsis or fungal invasion of critical organs secondary to refractory neutropenia are the usual causes of death in the modern era.
Reticulocytopenia and the absence of circulating blasts suggests aplastic anemia, which is confirmed by the fatty bone marrow biopsy. Despite the simple pathology, the differential diagnosis has become more difficult due to a profusion of advanced laboratory assays and an as yet uncertain nosology. Constitutional marrow failure, especially Fanconi anemia, can present in adulthood and without typical physical stigmata; this diagnosis is established by examination of chromosomes from mitotic peripheral blood cells after clastogenic stress. Sometimes a constitutional etiology is suspected despite normal chromosome studies, and a hereditary process must be inferred from a pedigree, physical signs such as the abnormal nails of dyskeratosis congenita, or the neurologic signs of the ataxia/pancytopenia syndrome. Among acquired diseases, pancytopenia with a hypocellular bone marrow can also result from aleukemic leukemia, lymphoma with marrow invasion, or large granular lymphocytic leukemia. The distinction between aplastic anemia and hypocellular myelodysplasia may be nearly arbitrary when it depends on too subtle morphologic features, and even abnormal marrow cytogenetics no longer exclude an immune-mediated marrow failure syndrome. As will be discussed below, paroxysmal nocturnal hemoglobinuria now is most often diagnosed concurrently with aplastic anemia.
Epidemiology
Large prospective studies indicate an annual incidence of two new cases per million population in Europe and Israel.1 The rate is much higher in the developing world, where aplastic anemia may rival acute myelogenous leukemia in frequency of diagnosis in hematology clinics; in formal studies in Thailand2 and China,3 the incidence has been determined to be about threefold that in the West. European studies have confirmed and quantified medical drugs as risks for the development of marrow failure (Table 1); surprisingly, drug use accounts for only a small fraction of the disease in Thailand, almost all of which is idiopathic.
A classification of acquired aplastic anemia.
|
|
Pathophysiology
The hallmark of aplastic anemia is the empty bone marrow, and by all measures hematopoiesis is markedly reduced.4 Not only are the distinctive hematopoietic precursor cells, the young forms of the erythroid and myeloid lineages and megakaryocytes, absent by visual examination of the aspirate and biopsy morphology, but imaging of the vertebrae shows uniform replacement of marrow with fat. CD34+ cells measured by flow cytometry are decreased in blood and marrow. Functionally, cells capable of forming erythroid, myeloid, and megakaryocytic colonies in semisolid media are much reduced, and in vitro assays of very primitive, quiescent hematopoietic cells, closely related to if not identical with true stem cells, show a similar consistent and severe deficit.5 Estimating from these assays, it is likely that patients with aplastic anemia present with pancytopenia when their stem cell and progenitors have fallen to -1% or less of normal numbers. While some blood cells are produced, evidence of the enormous compensatory capacity of the marrow, such a profound deficiency has important qualitative consequences, perhaps reflected in the shortened telomere length measured in granulocytes of patients with aplastic anemia,6 compatible with extremely stressed hematopoiesis.
The viability and differentiation of blood cell progenitors depend on specific hematopoietic growth factors, mainly produced by the marrow stroma. However, laboratory studies have generally shown normal function of aplastic anemia patients' stroma, and the circulating blood levels or in vitro production of almost all cytokines is normal and indeed elevated in the great majority of patients.7 Stromal dysfunction also appears unlikely from clinical observations; after bone marrow transplantation, many stromal elements remain of host origin, and growth factor administration is usually ineffective in patients with severe disease.
Certainly the commonest form of hematopoietic failure is iatrogenic—the transient aplasia that follows cytotoxic chemotherapy or radiation treatment. Benzene also has been thought to directly affect the marrow. However, patients with community-acquired aplastic anemia seldom have a history of such obvious exposures. The metabolism of common drugs can lead to the formation of toxic intermediate forms that can bind to protein, DNA, or RNA and lead to cellular injury; under certain circumstances, these metabolites have been assumed to accumulate specifically and harmfully in the marrow. Such a mechanism requires a potency for extremely low concentrations of metabolites equal to massive quantities of cancer chemotherapeutic agents specifically designed as cellular toxins and which in themselves do not usually lead to permanent marrow damage.
Clinical observations first suggested an alternative pathophysiology for marrow failure when Mathé reported unexpected blood count improvement in transplant patients who had rejected their grafts. He inferred that the conditioning regimen, which included an antilymphocyte serum to suppress the host's rejection response, had fortuitously treated an underlying autoimmune process.8 The efficiency of immune system destruction of hematopoiesis is also obvious in animal runt disease and in human transfusion-associated graft-versus-host-disease (GVHD); in these syndromes, small numbers of alloreactive T cells uniformly lead to fatal aplastic anemia.9 A large amount of laboratory data supports the hypothesis that, in most patients with acquired aplastic anemia, lymphocytes are responsible for the destruction of the hematopoietic cell compartment.10 Early experiments showed a suppressive effect of patients' lymphocytes on hematopoietic colony formation of normal persons and on the patients' own bone marrow. These cells produced a soluble inhibitory factor that ultimately was identified as γ-interferon, and indeed activation of a TH1-type T cell response was inferred from excessive production of interferon, tumor necrosis factor, and interleukin-2. Cytotoxic lymphocyte activation, and most recently the intracellular presence of type 1 cytokines,11 can be measured by flow cytometric methods in patients' blood and marrow. The result of this immune process is destructive, with Fas-mediated CD34+ cell death and activation of other intracellular pathways leading to cell cycle arrest and the release of nitric oxide. Immunity is local and has been modeled in cell culture systems in which low concentrations of γ-interferon are secreted into the marrow microenvironment. In an animal model of aplastic anemia, bone marrow failure is produced by injection of alloreactive lymphocytes, but pancytopenia can be prevented by treatment with a monoclonal antibody to γ-interferon.12 Acquired aplastic anemia appears to share an autoimmune pathophysiology with other human diseases in which TH1/TC1 cells effect organ-specific destruction, like multiple sclerosis, ulcerative colitis, uveitis, and type I diabetes.
Etiologies
Although most often aplastic anemia occurs without a suggestive prior history and is labeled idiopathic, in some cases a clear inciting event can be identified (Table 2). In contrast to sometimes nebulous histories of drug or chemical exposure, hepatitis has objective markers, and the post-hepatitis aplastic anemia syndrome has been well characterized clinically.13 Seronegativity for the usual viruses implicates a novel infectious agent (likely also responsible for fulminant hepatitis of childhood). Both laboratory and clinical studies suggest an immune pathophysiology, and the liver inflammation as well as marrow aplasia respond to immunosuppressive therapy.
Drugs associated with aplastic anemia in the International Aplastic Anemia Agranulocytosis Study.*
| Drug . | Stratified Risk Estimate# . | Multivariate Relative Risk Estimate# . |
|---|---|---|
| Nonsteroidal analgesics | ||
| butazones | 3.7 (1.9-7.2)# | 5.1 (2.1-12)# |
| indomethacin | 7.1 (3.4-15) | 8.2 (3.3-20) |
| piroxicam | 9.8 (3.3-29) | 7.4 (2.1-26) |
| diclofenac | 4.6 (2.0-11) | 4.2 (1.6-11) |
| Antibiotics | ||
| sulfonamides** | 2.8 (1.1-7.3) | 2.2 (0.6-7.4) |
| Antithyroid drugs | 16 (4.8-54) | 11 (2.0-56) |
| Cardiovascular drugs | ||
| furosemide | 3.3 (1.6-7.0) | 3.1 (1.2-8.0) |
| Psychotropic drugs | ||
| phenothiazines | 3.0 (1.1-8.2) | 1.6 (0.4-7.4) |
| Corticosteroids | 5.0 (2.8-8.9) | 3.5 (1.6-7.7) |
| Penicillamine | ∞ | |
| Allopurinol | 7.3 (3.0-17) | 5.9 (1.8-19) |
| Gold | 29 (9.7-89) |
| Drug . | Stratified Risk Estimate# . | Multivariate Relative Risk Estimate# . |
|---|---|---|
| Nonsteroidal analgesics | ||
| butazones | 3.7 (1.9-7.2)# | 5.1 (2.1-12)# |
| indomethacin | 7.1 (3.4-15) | 8.2 (3.3-20) |
| piroxicam | 9.8 (3.3-29) | 7.4 (2.1-26) |
| diclofenac | 4.6 (2.0-11) | 4.2 (1.6-11) |
| Antibiotics | ||
| sulfonamides** | 2.8 (1.1-7.3) | 2.2 (0.6-7.4) |
| Antithyroid drugs | 16 (4.8-54) | 11 (2.0-56) |
| Cardiovascular drugs | ||
| furosemide | 3.3 (1.6-7.0) | 3.1 (1.2-8.0) |
| Psychotropic drugs | ||
| phenothiazines | 3.0 (1.1-8.2) | 1.6 (0.4-7.4) |
| Corticosteroids | 5.0 (2.8-8.9) | 3.5 (1.6-7.7) |
| Penicillamine | ∞ | |
| Allopurinol | 7.3 (3.0-17) | 5.9 (1.8-19) |
| Gold | 29 (9.7-89) |
It is more difficult to confidently implicate a medical drug as a causative agent in an individual case. Aplastic anemia follows as a rare idiosyncratic reaction in perhaps 1/100,000 to 1/200,000 individuals exposed to a drug. Patients whose marrow failure has a presumed drug etiology are demographically identical and respond to treatment similarly to those with idiopathic aplastic anemia. The mechanism of drug-induced aplastic anemia is unknown and may involve specific metabolic pathways as well as aberrant immune responses.
Host factors have been suggested by higher specific histocompatibility antigen frequencies among patients. HLA-DR2 is about twice as frequent as in the normal population,14 and in Japanese patients a specific class II haplotype (DRB*1501) has been strongly associated with cyclosporine-responsive and -dependent disease.15 In one particularly well studied case, altered drug metabolism was incriminated as responsible for aplastic anemia in an epileptic man.16 However, why the immune response is set on a devastating pathologic course only in rare individuals exposed to a frequently used medication, or to a probably ubiquitous virus, is a central unresolved question.
Therapy
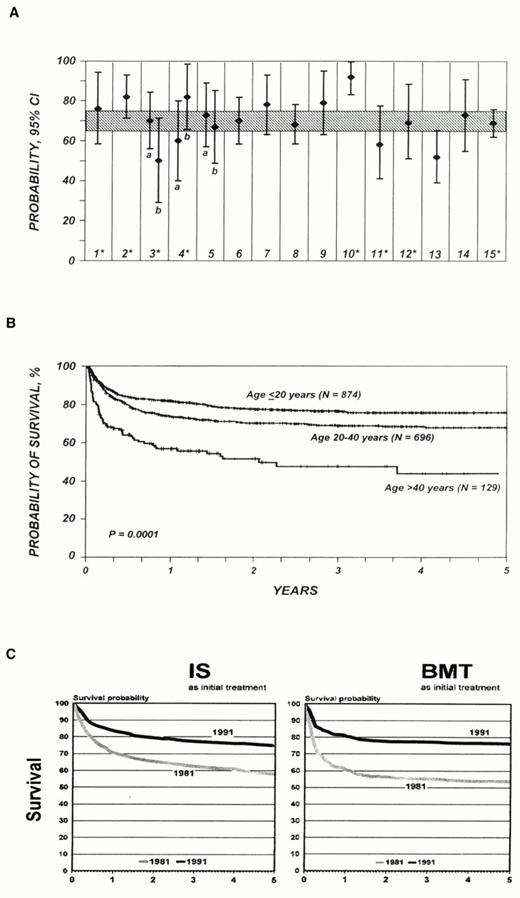
The underlying pathology of aplastic anemia can be addressed by replacing the marrow through stem cell transplantation or by quelling lymphocyte attack through immunosuppressive therapies.17,18 Bone marrow or, more recently, peripheral blood stem cell transplantation from a histocompatible sibling usually cures the underlying bone marrow failure. Survival rates have been reported as high as 90% from a single experienced institutions19 and at 75-80% for registry data, which reflect more general experience20,21 (Figure 1A, B). Death rates for the first 100 days post transplant have fallen, probably due to lower rates of graft rejection and better control of infections. GVHD, the frequency and severity of which correlate with patient age, continues to limit the success of transplantation, largely accounting for the lower survival of adults compared to children in most analyses (Figure 1C).22 In the most recently published Seattle data, 41% of 212 patients who had survived more than 2 years after transplant suffered chronic GVHD, and their mortality rate was three times higher than for patients without this complication23 (and of course GVHD contributed to earlier deaths as well). Efforts to address GVHD in aplastic anemia by T cell depletion of the marrow inoculum were disastrous, resulting in high rates of primary graft failure.
(A) Survival after allogeneic bone marrow transplant: data from individual hospital series in peer-reviewed publications, 1991-1997 (1-15, with 95% confidence intervals); shaded area represents 5-year survival (with the same confidence intervals) of patients reported to the International Bone Marrow Transplant Registry during the same time period (reprinted from21 , which provides details).
(B) The continuing influence of age on survival as reflected in IBMTR data (reprinted from21 ).
(C) Comparative probability of survival after immunosuppression (IS) and bone marrow transplant (BMT) for patients treated in the 1980s and 1990s, from the Working Party on Severe Aplastic Anemia of the European Blood and Marrow Transplant Group (reprinted from51 ).
(A) Survival after allogeneic bone marrow transplant: data from individual hospital series in peer-reviewed publications, 1991-1997 (1-15, with 95% confidence intervals); shaded area represents 5-year survival (with the same confidence intervals) of patients reported to the International Bone Marrow Transplant Registry during the same time period (reprinted from21 , which provides details).
(B) The continuing influence of age on survival as reflected in IBMTR data (reprinted from21 ).
(C) Comparative probability of survival after immunosuppression (IS) and bone marrow transplant (BMT) for patients treated in the 1980s and 1990s, from the Working Party on Severe Aplastic Anemia of the European Blood and Marrow Transplant Group (reprinted from51 ).
Allogeneic transplantation is available only to a minority of patients, as about 70% will lack a suitably matched sibling donor. Phenotypically identical alternative family donors are acceptable but are found for only an occasional patient. Many more donors are available outside the family and can now be located through large registries in the United States and Europe. Relatively good results have been achieved at Children's Hospital in Milwaukee, where T cell depletion of the graft is combined with cytosine arabinoside, cyclophosphamide, and total body irradiation; survival at a median follow-up of about three years in 28 children was 54%, despite the heavy transfusion burden and previous treatment, and GVHD was infrequent.24,25 Results elsewhere have been more disappointing, especially among adults, owing to high rates of graft rejection, GVHD, and infection caused by delayed immune system reconstitution; in general, survival has been about half that observed with standard transplants, 29%26 to 34%.27 The rigorous conditioning regimens required for engraftment are poorly tolerated by older patients and, even among children, seem likely to exact a delayed toll in late malignant disease. In aplastic anemia, malignant tumors occur at a higher than expected rate among patients undergoing standard conditioning;28,29 intensive chemo- and radiotherapies used in unrelated donor regimens would be predicted to eventually result in higher rates.30
Immunosuppression is employed in patients who are not candidates for stem cell transplantation due either to age or the lack of a donor. Horse antithymocyte globulin (ATG) and rabbit antilymphocyte globulin (ALG) are now both licensed for use in the United States. Hematologic responses, which are usually defined as sufficiently improved blood counts such that the patient no longer requires transfusions of red blood cells or platelets and is not susceptible to infection, occur in 40-50% of those treated with either ATG or ALG alone.17,31 For patients with severe aplastic anemia, the addition of cyclosporine to ATG or ALG has improved response and survival rates. In European32 and American33 studies, response rates have been 70-80%, and survival at 5 years among responders is about 90%. Combined treatment with cyclosporine and ATG has been particularly beneficial for children and patients with absolute neutropenia, compared with results for ATG alone. However, cyclosporine as a single agent of immunosuppression is inferior to ATG or ALG.34
ATG and ALG have distinctive toxicities. As foreign proteins, they can elicit anaphylaxis in the host; we routinely skin test for evidence of sensitivity and desensitize patients who have a positive reaction. ATG is not specific for lymphocytes and can reduce already low platelet and neutrophil levels and cause a positive direct antiglobulin test. Antibodies produced by the patient to horse proteins can lead to immune complex formation and serum sickness, usually about 11 days after initiation of treatment.35 Cyclosporine is nephrotoxic, and both serum creatinine and drug levels should be monitored to avoid irreversible renal damage; hypertension, gingival hyperplasia, and gastrointestinal and neurologic symptoms are other common side effects, and because of the risk of Pneumocystis carinii, we administer prophylaxis with monthly pentamidine inhalations.
Many (perhaps most) patients with aplastic anemia are not adequately treated by a single 4-day course of ATG followed by 6 to 12 months of cyclosporine. Slowly declining blood counts signal a need to reinstitute treatment and usually respond to either an increase in the dose or the reinstitution of cyclosporine. Some patients appear to depend on continued administration of cyclosporine, often at relatively low doses. Frank pancytopenia can recur and prompt a second course of ATG. However, long-term prognosis does not appear to be affected by relapse. Patients who respond to immunosuppression often continue to have blood counts that, while adequate for full activities, remain below normal. Incomplete responses, frequent relapses, and cyclosporine-dependence are probably evidence of chronic immune system activity on a hematologically compensated bone marrow. A further problem is the development of late clonal diseases (see below).
Small numbers of patients at Johns Hopkins were treated with cyclophosphamide at high doses equivalent to those employed in transplant conditioning regimens but without stem cell rescue, administered during intervals in the 1980s when ATG was temporarily not available; of the seven responding patients, six had normal blood counts, never had relapsed, and were without clinical evidence of clonal disease.36 Based on this report, we initiated a randomized trial to compare ATG to high dose cyclophosphamide; patients in both arms received cyclosporine. However, this study was terminated prematurely due to excess toxicity, mainly severe fungal infections, and deaths in the group that received cyclophosphamide.37 High-dose cyclophosphamide is a much more aggressive form of immunosuppression than antilymphocyte sera and results in profound and sustained suppression of bone marrow function as well; profound, virtually absolute neutropenia can persist for many weeks, necessitating prolonged courses of antibiotics and even granulocyte transfusions, and several of our patients were iatrogenically converted from severe to super-severe disease, with its associated poorer immediate prognosis. Whether cyclophosphamide's putative advantages are worth such acute risks seems doubtful, especially when other therapeutic approaches are promising.
One such strategy is based on induction of tolerance. ATG and ALG reduce lymphocyte numbers, but transiently and modestly compared with cytotoxic chemotherapy. Part of their beneficial activity may be to induce tolerance, perhaps by specific deletion of activated lymphocytes; indeed, the concurrent use of cyclosporine, which blocks T cell activation, may blunt ATG's effects.38,39 In our new NIH protocol for severe aplastic anemia, we have delayed the introduction of cyclosporine and added a novel immunosuppressive drug, mycophenolate mofetil; mycophenolate, by inhibiting inosine monophosphate, is cytotoxic for cycling T cells. Activated lymphocytes should be subject to elimination by their characteristic cell surface antigens (recognized by ATG) and their mitotic activity. To date, 21 patients have entered this protocol; mycophenolate's activity has been dramatically apparent in the early and substantial increases in neutrophil counts in patients with extremely severe disease. Mycophenolate lacks nephrotoxicity, and its use may also allow decreased doses and earlier termination of cyclosporine as well as prevent relapse. Other milder but more specific forms of immunosuppression might also be effective. For example, ATG contains antibody specificities for the interleukin-2 receptor, present on activated lymphocytes; we are testing daclizumab (Zenapax®), a commercially available monoclonal antibody to the receptor in patients with moderate aplastic anemia. Other monoclonal antibodies, recombinant soluble cytokine receptors, and new immunosuppressive drugs like rapamycin deserve examination in immune-mediated marrow failure syndromes.
There are no clear guidelines for the treatment of refractory aplastic anemia. Multiple courses of immunosuppression are commonly administered at European centers: the majority of patients who received rabbit ALG after failing horse ALG became transfusion-independent in a recent Italian study.40 Androgens in various formulations have been employed in aplastic anemia for many decades and they are occasionally effective, particularly if hematopoietic failure is not complete,41,42 their mechanism of action in aplastic anemia is not well understood but may relate to immunomodulation rather than to effects on erythropoietin production or on hematopoietic cells. There is little justification for either a therapeutic trial of corticosteroids as primary treatment or their chronic use to prevent bleeding; aplastic anemia patients appear particularly susceptible to aseptic necrosis as a complication of steroid use.43 Therapeutic trials of hematopoietic growth factors are not appropriate as first-line treatment of severe aplastic anemia (G-CSF and GM-CSF can increase granulocyte counts in aplastic anemia, but this effect is almost always transient and rarely occurs in patients with very severe neutropenia).44 G-CSF also does not improve the response rate or survival in patients undergoing standard immunosuppressive treatment.45 However, growth factors can be useful in refractory aplastic anemia, with clinically meaningful improvement in blood counts after prolonged administration of G-CSF, erythropoietin, and stem cell factor, usually in some combination.46,47,48 While in Japan chronic G-CSF therapy has been linked to the development of the dire cytogenetic abnormality monosomy 749,50 (see below), this dangerous relationship has not been reported elsewhere.
Only a few patients face an actual choice between allogeneic transplantation and immunosuppressive therapy. Analyses of large databases have not shown major differences in outcomes between these two therapeutic approaches.51 Nevertheless, transplant is probably preferable for certain defined subgroups, especially young patients and those with very severe neutropenia. Patients who failed immunosuppressive therapy have later undergone successful transplantation from matched siblings or from unrelated donors.
Late Evolution
Aplastic anemia is closely related to other bone marrow failure syndromes. Myelodysplasia, paroxysmal nocturnal hemoglobinuria (PNH), and aplastic anemia are easily confused in the clinical classification of a patient with pancytopenia and a hypocellular bone marrow, and they may indeed share common pathophysiologic features. As patient survival has improved, so too has the opportunity to observe the chronic course of marrow failure. Evolution of aplastic anemia has been reported in a substantial minority of patients undergoing immunosuppressive therapy: in a large European series of more than 200 patients, the actuarial risk of developing PNH was 13% at 7 years, and 15% for myelodysplasia and leukemia.52 In our series of more than 100 patients followed at the NIH Clinical Center, the actuarial risk for all late clonal evolution was 16% at 7 years. Immunosuppression itself is not etiologic, as similar data have been acquired in patients treated with androgens.42 Myelodysplastic marrow changes and cytogenetic abnormalities tended to appear late, sometimes years after initiation of therapy, and often (but not always) in the setting of a poor response to treatment. In contrast, PNH was usually diagnosed within the first or second year in patients who had shown improvement in blood counts, and often was purely a laboratory finding.
Indeed, whether “evolution” accurately describes the relationship among these diseases is questionable. For the diagnosis of PNH, highly sensitive flow cytometric tests, which detect the absence of those proteins anchored to the cell surface by glycosylphosphoinositol anchors, has replaced the classic Ham test. By flow cytometry, a large proportion of bone marrow failure patients at the time of presentation show deficient granulocytes. In our series, 15% of aplastic anemia patients and 23% of myelodysplasia patients showed PNH cells early in their disease and before any treatment.53 Clones also disappear over time, especially those present at low levels initially. Of interest will be comparable analyses utilizing more sensitive assays to detect chromosomal abnormalities typical of myelodysplasia, such as fluorescent in situ hybridization. Patients with myelodysplasia, especially of the refractory anemia subtype, can show blood count improvement with the same immunosuppressive treatments employed in aplastic anemia.54 One tenable unifying hypothesis is that both PNH and myelodysplasia represent clonal stem cell escape from immune system attack.
Supportive Care
Anemia and thrombocytopenia can be corrected by transfusion. Limited numbers of blood transfusions probably do not affect the outcome of stem cell transplantation. Red blood cells should be infused to achieve hemoglobin levels compatible with full activity, usually above 70 gr/L (90 gr/L in patients with cardiopulmonary compromise). Platelet collection by cytopheresis and leucocyte reduction by ultraviolet light or filtration are measures that reduce alloimmunization from transfusions.55 The long-term benefit of a prophylactic platelet transfusion program is unclear; when patients require periodic platelet administration to control hemorrhage, maintenance of levels above 10 × 109/L is adequate. Neutropenic fevers must be treated aggressively with parenteral, broad-spectrum antibiotics, and antifungal therapy should be added for persistent fever. Attention to details of oral hygiene and hand washing and avoidance of minor injuries or casual exposure to infectious agents can reduce the risk of serious complications. Good medical practice also requires a relationship between physician and patient that provides for honest discussion of therapeutic options, prompt response to crises, and psychological sustenance. Frightening symptoms, delayed or absent improvement in blood counts, relapses, and complications of therapy must be dealt with realistically but also reassuringly. Even refractory patients occasionally make unexpected and sustained recoveries.
Conclusions
In the last century, both our understanding of the origin of aplastic anemia and the definitive and supportive treatment of the individual patient have improved enormously. Aplastic anemia is usually immunologically mediated, sharing pathophysiologic mechanisms with other autoimmune diseases. Unfortunately, stem cell destruction may be quite advanced by the time the patient presents with pancytopenia. Replacement of hematopoietic tissue by either stem cell transplantation or suppression of the pathologic immune response to allow recovery of the patient's own marrow are both effective. Improvements in methods for monitoring hematopoiesis and immune system activity and especially in the application of immunomodulatory drugs should be of clinical benefit. Major research questions remain as to the nature of inciting antigens and the determinant of the aberrant immune response, as well as the fundamental pathophysiologic relationship among aplasia, dysplasia, and paroxysmal nocturnal hemoglobinuria.
II. Acquired Pure Red Cell Aplasia: Physiology and Therapy
Janis L. Abkowitz, M.D.*
Hematology Division, University of Washington, Box 357710, Seattle WA 98195-7710
Acquired pure red cell aplasia (PRCA) is characterized by severe anemia, reticulocytopenia, and the absence of hemoglobin-containing cells in an otherwise normal marrow aspirate. It can occur independently or in association with systemic disorders, including lymphoma and rheumatologic disease. Sometimes, proerythroblasts persist, while in other circumstances the block in erythroid differentiation occurs earlier. With cell culture studies, the level of differentiation that is impaired can be defined as prior to burst-forming unit erythroid (BFU-E) (23% of the 35 cases in one study1 ), BFU-E to colony-forming unit erythroid (CFU-E) (29% of cases), CFU-E to proerythroblast (31% of cases), and after proerythroblast (17% of cases). As PRCA involves a single (erythroid) lineage in a stage-specific fashion, studies of this disorder provide insights into the physiology of differentiation, and specifically those events that are important for erythropoiesis.
There are three etiologies of PRCA: 1) immunologic, 2) viral (human parvovirus B19 [B19]), and 3) myelodysplasia. Understanding the etiology of PRCA is important for clinical decision making and implementation of correct therapies.
Immunologic PRCA
Most cases of PRCA can be attributed to immunologic interactions. PRCA has been associated with thymoma, drugs such as azathioprine and procainamide, rheumatoid arthritis, systemic lupus erythematosis (SLE), hepatitis, mononucleosis, lymphoma, B cell chronic lymphocytic leukemia (CLL), T cell CLL, large granular lymphocytic (LGL) leukemia, and angioimmunoblastic lymphadenopathy.1,2,3,4 Although these are a diverse group of diseases, there are common pathophysiologies linking them and red cell failure. Antibodies have been described in patients' sera that are selectively cytotoxic for marrow erythroid cells or are directed against erythropoietin.2,5,6 T cells from patients with PRCA associated with thymoma,2,7 chronic Epstein-Barr viral infection,8 lymphoma,9 CLL,10,11,12 and LGL leukemia3,4,13,14 have been shown to suppress erythropoiesis in vitro. The anemia in patients with red cell aplasia associated with these disorders generally responds to immunosuppressive therapies, including corticosteroids, cyclosporine, and low-dose cyclophosphamide.1,3,15
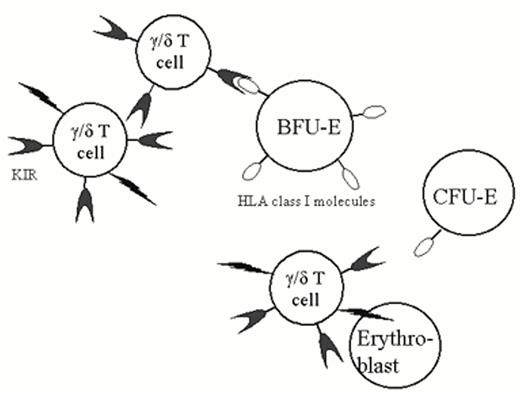
A particularly instructive case was recently described by Rupert Handgretinger and colleagues.4 This 56-year-old man developed PRCA in the setting of LGL leukemia. The large granular lymphocytes were defined as γ/δ T-cells by flow cytometry (the rearranged receptor variable-region genes were Vγ4 and Vδ1). They were CD2, CD3, CD5, and CD7 positive and failed to express antigens characteristic of natural killer (NK) cells (CD16 and CD56). BFU-E and CFU-E were observed in marrow culture studies, but proerythroblasts were absent on the marrow aspirate, suggesting that the block in erythropoiesis occurred as CFU-E matured to proerythroblasts. In subsequent studies, these investigators demonstrated that the γ/δ T-cells lysed target cells in an MHC unrestricted manner. However, as these cells also expressed killer-cell inhibitory receptors (KIR), target cells that display HLA class I proteins were spared. Although all normal early hematopoietic progenitor cells express HLA class I determinants, surface class I antigen expression is downregulated as CFU-E mature to proerythroblasts. The authors demonstrated that at this stage of differentiation, erythroid cells became sensitive to lysis by the expanded (clonal) population of γ/δ T-cells, resulting in the phenotype of pure red cell aplasia (Figure 2). It is possible that comparable mechanisms will be implicated in the patho-physiology of other autoimmune disorders.
The patient's γ/δ T cells express KIR (killer inhibitory receptors). When KIR bind HLA class I antigens on the surface of a target cell, the ability of the effector cell to lyse its target is inhibited. Normal BFU-E express class I determinants, but this expression is down-regulated as CFU-E mature proerythroblasts. This patient had a clonal expansion (LGL leukemia) involving γ/δ T cells, resulting in the “autoimmune” lysis of proerythroblasts and PRCA.
The patient's γ/δ T cells express KIR (killer inhibitory receptors). When KIR bind HLA class I antigens on the surface of a target cell, the ability of the effector cell to lyse its target is inhibited. Normal BFU-E express class I determinants, but this expression is down-regulated as CFU-E mature proerythroblasts. This patient had a clonal expansion (LGL leukemia) involving γ/δ T cells, resulting in the “autoimmune” lysis of proerythroblasts and PRCA.
There are many therapeutic options for immunologic PRCA. Patients generally respond to steroids, cyclosporine, cyclophosphamide, or antithymocyte globulin (ATG).1,2,15,16 Responses to androgens, plasmapheresis, and splenectomy have been described.15,16 Treatment of an underlying lymphoproliferative disorder with combination chemotherapies or fludarabine16 can lead to a remission of PRCA, but this is not often required. An independent clinical decision should be made about the appropriateness of treating the underlying disorder, and the treatment of PRCA should be approached as one would approach the treatment of idiopathic thrombocythemia purpura (ITP) or hemolytic anemia complicating stage 0 CLL. We generally begin with a therapeutic trial of prednisone (1 mg/kg/d po x 4-6 weeks) and follow the transfusion interval, reticulocyte count, and hematocrit as the indication of response. If this is unsuccessful (only 27-37% of patients appear to respond1,15 ), we begin a second-line agent, either cyclophosphamide (75-100 mg po qd in adults, decreased if needed to maintain the granulocyte count > 1.5-2.0 × 103/ml) or cyclosporine (standard dosing). For each drug, we allow a 8-10 week trial. Because red cell transfusion is of low morbidity, we have chosen this approach rather than one with more aggressive additional therapies. In patients with underlying systemic lupus erythematosus (SLE) or rheumatologic disorders, we generally use cyclophosphamide as the second agent. In children and in individuals with B cell CLL cyclosporine is used.6,18 We continue with sequential treatment trials, including ATG,19 until we find that agent to which the patient responds. Responding individuals are treated for 3-6 months. Most do not relapse with discontinuation of therapies. In fact, of 24 patients that achieved complete response in the studies of reference 1, 20 (83%) had a normal hematocrit (without continued therapy) at the time (median 5 years) of follow-up observations. In patients with LGL leukemia of an NK cell phenotype, low-dose methotrexate (i.e., 15 mg po q week) is often efficacious.1
Viral PRCA
A second physiology of PRCA is viral-induced disease. Human parvovirus B19 (B19) (reviewed in 20 ) is a common viral disorder, usually acquired early in life. By age 15, 50% of children have detectable IgG to B19, confirming prior exposure, and infection is prevalent, so that 90% of the elderly are seropositive.21 B19 is the cause of fifth disease in young children and of an arthropathy syndrome in adults. B19 specifically infects and lyses erythroid precursor cells.20 The cellular receptor is the blood group P antigen, globoside, which is expressed on some CFU-E and all proerythroblasts and subsequent erythroid cells.22 B19 infection can produce characteristic giant proerythroblasts with eosinophilic nuclear inclusion bodies in marrow aspirates.20 This morphologic feature, however, can also be seen in patients with HIV-1 in the absence of B19 infection.23
In a patient with a chronic hemolytic anemia (e.g., hereditary spherocytosis, sickle cell anemia), B19 infection results in profound anemia (an acute PRCA), also termed aplastic crisis. It is likely that all individuals who acquire B19 will develop marrow manifestations consistent with PRCA. However, an immunologically normal host clears this viral infection and recovers normal hematopoiesis within a few weeks. As the lifespan of a red cell is 120 days, anemia never develops. Because of the short circulatory red cell lifespan in patients with chronic hemolysis, severe anemia quickly occurs. Symptomatic anemia can be treated with red cell transfusions. B19 infection will (because of the intact immune status of the host) spontaneously resolve and the patient's hematocrit return to its baseline value. Patients with chronic immunologic abnormalities, including HIV-1 infection, may be unable to resolve B19 infection.23 In this circumstance, an erythroid marrow failure, clinically indistinguishable from idiopathic or other secondary forms of PRCA, develops (Table 3).
Human parvovirus B19 infection and anemia.
| Patient . | RBC Lifespan . | Immune Status . | Time Required to Mount an Effective Immune Response . | Outcome . |
|---|---|---|---|---|
| Normal individual | 120 d | Normal | 14-21 d | Normal Hct is maintained |
| Chronic hemolysis | 5-10 d | Normal | 14-21 d | Acute (transient) PRCA |
| HIV | 120 d | Abnormal | Unable to clear B19 | PRCA |
| Erythropoiesis is suppressed in all patients infected with B19. Recovery occurs at 14-21 days (as B19 is cleared) in those individuals with normal immune function. Patients with chronic hemolysis develop a severe (transient) anemia, termed “aplastic crisis,” because their RBC lifespan is short. Patients with HIV may be unable to clear B19 and thus can develop anemia after 2-4 months. | ||||
| Patient . | RBC Lifespan . | Immune Status . | Time Required to Mount an Effective Immune Response . | Outcome . |
|---|---|---|---|---|
| Normal individual | 120 d | Normal | 14-21 d | Normal Hct is maintained |
| Chronic hemolysis | 5-10 d | Normal | 14-21 d | Acute (transient) PRCA |
| HIV | 120 d | Abnormal | Unable to clear B19 | PRCA |
| Erythropoiesis is suppressed in all patients infected with B19. Recovery occurs at 14-21 days (as B19 is cleared) in those individuals with normal immune function. Patients with chronic hemolysis develop a severe (transient) anemia, termed “aplastic crisis,” because their RBC lifespan is short. Patients with HIV may be unable to clear B19 and thus can develop anemia after 2-4 months. | ||||
Chronic B19 infection should be considered as an etiology for PRCA in all individuals with HIV-1 infection (independent of the stage of disease),23,24,25 as well as in individuals with other immunologic impairments (congenital immunodeficiency or immunodeficiency secondary to cytotoxic or immunosuppressive drugs).26 PRCA due to persistent B19 has been reported in patients without clinically evident immunodeficiency.26 It is important to diagnose PRCA secondary to B19 infection, because patients are uniformly responsive to immunoglobulin therapy.20 ,23,24,25,26 Because of the high prevalence of B19 in the general population, commercial sources of IgG contain high titers of antibodies to the virus. Therapy with IgG (dose options equivalent to ITP therapy) results in a reticulocytosis within 3-5 days and the subsequent resolution of the anemia. B19 titers decrease from 1012 B19 DNA copies/ml serum to 106 (i.e., detectable only by PCR) in HIV-1-positive patients, but the infection likely never totally resolves.23 Therefore, these individuals sometime recur with PRCA 6-9 months later and respond to the readministration of IgG.23,24,25,26
To diagnose B19 as the cause of PRCA, the appropriate study is a dot blot analysis of serum (see discussion in reference25 ). PCR studies may be too sensitive as treated (and responding) HIV-1-positive patients have persistent PCR positivity, and normal individuals may remain positive for B19 by PCR for many months after clearing clinical infection.20,21 Thus, results could be falsely positive if obtained at a time of a clinical epidemic. Currently, DNA analyses are not commercially available. Therefore, we often use the standard PCR assay (available in many reference laboratories) as a screening test. If this is negative, the patient does not have B19 as the etiology of PRCA. If it is positive, one needs to consider the cost/benefit of a IgG treatment trial versus a confirmatory assay by DNA hybridization (available in the research setting).
Although B19 is the only described viral cause of human PRCA, feline leukemia virus subgroup C/Sarma (FeLV-C) has been identified as the virologic agent responsible for PRCA in cats. This animal model of PRCA, is of interest from a physiologic standpoint. FeLV-C appears to block BFU-E to CFU-E differentiation by impairing the cell surface expression or function of the retroviral receptor. Quigley et al27 and Tailor et al28 have recently identified the feline and human FeLV-C receptors (FLVCR), respectively. The receptor cDNA is predicted to encode a 560 amino acid protein with 12 membrane-spanning domains that shares homology with receptor for D-glucarate (an anionic sugar) in C. elegans and bacteria. It is likely that this molecule will have a specific role in erythroid differentiation, and that understanding its action will provide insights into events that are critical to the normal maturation of erythroid cells from BFU-E to CFU-E.
PRCA as the Initial Presentation of Myelodysplasia
Myelodysplasia is a clonal disorder originating in hematopoietic stem or early progenitor cells. Generally, all three lineages (red cells, white cells, platelets) are affected and the marrow is hyperproliferative and dysmorphic. A variant of myelodysplasia has been described in which the neoplastic progenitor cell has a limited differentiation capacity, leading to a clinical phenotype that is indistinguishable from aplastic anemia.29 Similarly, in rare circumstances, PRCA can be the only or initial manifestation of myelodysplasia.1,3,30 The anemia is often unresponsive to immunosuppressive agents.1,3,30 In marrow culture studies, BFU-E are not detected, suggesting that the origin of this disease is in a multipotent stem/precursor cell that cannot undertake the erythroid differentiation pathway.1,31 Sometimes there are subtle dysmorphic features of granulocytes (e.g., a Pelger-Huet abnormality), the presence of excess basophils, or unexplained marrow fibrosis that raise the suspicion of myelodysplasia. However, the marrow may be indistinguishable from that of typical acquired PRCA.1 Perhaps the simplest diagnostic study is cytogenetics. An abnormal marrow karyotype (in the absence of leukemia/lymphoma involving the marrow) establishes this diagnosis.1,3,30
A Clinical Approach to Patients with Acquired PRCA
In patients with profound anemia and reticulocytopenia, yet with normal platelet and white blood cell counts, we obtain a marrow aspirate and biopsy to establish a diagnosis of PRCA. If lymphoid cells are increased in number in the blood or marrow or demonstrate aberrant morphology (i.e., LGL), immunophenotyping and studies of clonality by T cell or immunoglobulin gene rearrangement (when appropriate) are obtained. We do a careful physical examination and history and obtain blood studies if needed to consider the diagnosis of associated disorders such as SLE or rheumatoid arthritis. Although thymoma is uncommonly associated with PRCA, we generally check a chest x-ray or computerized tomogram (CT). A history of thymoma, concurrent thymoma, or the subsequent development of thymoma is reported in 8% and 5% of PRCA patients in two large series.1,15 Earlier frequency estimates (30-50%; derived from the analysis of case presentations) reflect significant reporting bias. As most patients respond to prednisone or a second line agent (e.g., cyclophosphamide, cyclosporine), we generally initiate treatment trials prior to any further investigations such as marrow culture studies or cytogenetics. In refractory patients with normal cytogenetics and where serum does not contain B19 DNA, culture studies can be informative (Figure 3). If erythroid bursts are present in normal numbers, it implies that BFU-E are present in the patient and, when removed from serum antibody and the patient's T (or NK) cells, can mature into hemoglobinized colonies. This finding justifies sequential therapies with additional immunologic agents. In general, PRCA is a very treatable disorder (80% response rate1 ), as well as one that provides unique insights into the physiology of erythroid differentiation.
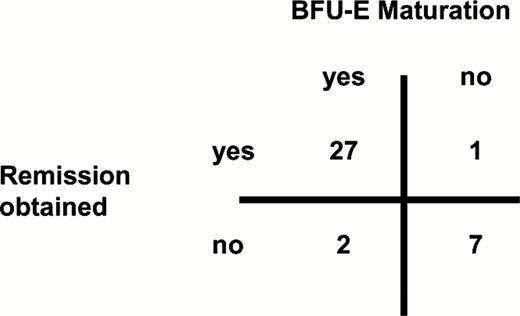
The clinical value of in vitro culture.
The relationship of normal BFU-E growth (30 bursts/105 marrow mononuclear cells) and remission (CR and PR) is shown. BFU-E maturation in vitro was a superb predictor of clinical response. Its sensitivity was 96%, its specificity was 78% and its predictive value was 93% (p =.0001 with 2-tailed chi-square analysis). Complete data are in reference 1 .
The clinical value of in vitro culture.
The relationship of normal BFU-E growth (30 bursts/105 marrow mononuclear cells) and remission (CR and PR) is shown. BFU-E maturation in vitro was a superb predictor of clinical response. Its sensitivity was 96%, its specificity was 78% and its predictive value was 93% (p =.0001 with 2-tailed chi-square analysis). Complete data are in reference 1 .
III. Paroxysmal Nocturnal Hemoglobinuria
Lucio Luzzatto, M.D.*
Department of Human Genetics, Memorial Sloan Kettering Cancer Center, 1275 York Avenue, New York, NY 10021, USA; and Istituto Nazionale Ricerca sul Cancro, IST, Largo Rosanna Benzi 10, Genova, Italy.
The phrase paroxysmal nocturnal hemoglobinuria reflects the most dramatic manifestation of a blood disorder that we must now regard as quite complex. Indeed, hemoglobinuria follows hemoglobinemia, which in turn results from intravascular hemolysis; therefore, PNH has been traditionally and correctly classified as a hemolytic anemia. However, often the anemia is associated also with a decrease in neutrophils, or platelets, or both, hinting to a broader pathology of the hematopoietic system. In addition, patients with PNH are liable to the potentially devastating consequence of venous thrombosis, particularly in the veins of the porto-hepatic system. The triad of hemolytic anemia, pancytopenia and thrombosis makes PNH a truly unique clinical syndrome (see Table 4).
PNH: clinical heterogeneity and proposed terminology (from 40 ).
| Predominant Clinical Features . | Blood Findings . | Size of PNH Clone . | Designation . |
|---|---|---|---|
| Hemolysis ± thrombosis | Anemia; little or no other cytopenia | Large | Florid PNH |
| Hemolysis ± thrombosis | Anemia; mild to moderate other cytopenia(s) | Large | PNH, hypoplastic |
| Purpura and/or infection | Moderate to severe pancytopenia | Large | AA/PNH |
| Purpura and/or infection | Severe pancytopenia | Small | AA with PNH clone |
| Thrombosis | Normal or moderate cytopenia(s) | Small | Mini-PNH |
| Predominant Clinical Features . | Blood Findings . | Size of PNH Clone . | Designation . |
|---|---|---|---|
| Hemolysis ± thrombosis | Anemia; little or no other cytopenia | Large | Florid PNH |
| Hemolysis ± thrombosis | Anemia; mild to moderate other cytopenia(s) | Large | PNH, hypoplastic |
| Purpura and/or infection | Moderate to severe pancytopenia | Large | AA/PNH |
| Purpura and/or infection | Severe pancytopenia | Small | AA with PNH clone |
| Thrombosis | Normal or moderate cytopenia(s) | Small | Mini-PNH |
Epidemiology
PNH is encountered in all populations and can affect people of all ages and socio-economic groups. However, PNH has never been reported as a congenital disease, and there is no report of family clustering. Thus, PNH is an acquired disease. There is little information on the incidence of PNH, but the rate is estimated to be 5-10 times less than that of aplastic anemia; thus, PNH is a rare disease. It has been suggested that, like aplastic anemia, PNH may be more frequent in South East Asia and in the Far East.1
Clinical Features
In most cases2 the patient presents as a problem in the differential diagnosis of anemia, whether symptomatic or discovered incidentally. The diagnosis is immediately made much easier if the patient reports having passed dark urine, which is due to hemoglobinuria, the landmark of intravascular hemolysis. In some patients PNH may present with typical signs and symptoms of thrombosis in a deep vein in one of the limbs; in others with recurrent attacks of severe abdominal pain, which may prove to be due to intra-abdominal thrombosis. The classic site is in the hepatic veins, causing the Budd-Chiari syndrome, but the mesenteric vessels, the splenic, or the portal vein may be also involved. Not infrequently, the anemia is associated with other cytopenias, suggesting some degree of bone marrow failure (BMF). Indeed, sometimes a PNH patient may become less hemolytic and more pancytopenic, converting in fact to aplastic anemia. Conversely, PNH can emerge in patients with a previous diagnosis of aplastic anemia; indeed, in some recent series small PNH clones have been detected in up to 50% of cases of aplastic anemia.3
The natural history of PNH is that of a very chronic disorder, which may afflict the patient continuously for decades. Without treatment the median survival is estimated to be about 8 years; in the past the most common causes of death have been thrombosis or hemorrhage associated with severe thrombocytopenia. Rarely, PNH may terminate in acute myeloid leukemia. On the other hand, full spontaneous recovery from PNH also has been well documented.4
Hemolysis
Hemolysis in PNH is due to an intrinsic abnormality of the red cell. This defect was first characterized serologically through the finding that, unlike in auto-immune or allo-immune hemolytic anemia, there was no specific antibody involved. Rather, the red cells hemolyze whenever they are in the presence of activated complement (C), whether it is activated by an antibody (as with anti-I, present at low titer in most normal people), or through the alternative pathway (by lowering pH or ionic strength). Indeed, acidification of serum was used to develop a diagnostic test that is still in use in laboratories,5,6 and low ionic strength has been used for a screening test (sucrose hemolysis), which should be regarded as obsolete. It took half a century before the biochemical basis for this peculiar hypersusceptibility to C was clarified. We now know that several membrane proteins protect cells—including red cells—against damage from the membrane attack complex of C (C5-C9). One of these proteins, CD59, specifically hinders the insertion into the membrane of C9 polymers.7,8 CD59 is severely deficient or completely absent from the membrane of PNH red cells.9 This explains why there is chronic hemolysis in PNH, why the hemolysis is largely intravascular, and why it can be dramatically exacerbated in the course of a viral or bacterial infection, when antigen-antibody reactions associated with the infection will cause bursts of C activation.
Biochemical Abnormalities and Somatic Cell Mosaicism
Serological studies had suggested long ago that certain normal antigens were poorly expressed on the surface of PNH red cells. With the introduction and increasing use of flow cytometry, it was discovered that a bewildering multitude of membrane proteins is deficient in the abnormal population of blood cells of different lineages in patients with PNH.10 In most cases there is no obvious similarity in the function of these proteins; rather, they have a common structural element, namely a phospholipid moiety: a glycosyl phosphatidyl inositol (GPI) that includes an ethanolamine which can form a peptide link with a C-terminal amino acid of certain proteins. Because GPI is embedded in the lipid bilayer of the membrane, and it serves to retain the protein attached to the membrane, it is referred to as a GPI anchor. The fact that all GPI-linked proteins are deficient on the membrane of PNH cells suggested that the underlying defect might be in the complex biochemical pathway through which the GPI is synthesized in mammalian cells.
A remarkable feature of patients with PNH, when compared with those who have hemolytic anemia due to some other intracorpuscular cause, is that not all their red cells participate in the hemolytic process and that some erythrocytes are qualitatively normal. This observation led to the idea that PNH is a clonal disorder due to a somatic mutation.11 Direct supporting experimental evidence was obtained three decades ago in PNH patients who were heterozygous for the X-linked gene encoding glucose 6-phosphate dehydrogenase12 and in whom all PNH cells expressed the same G6PD allele. Thus, we have true somatic cell mosaicism in patients with PNH, complicating efforts to identify the biochemical abnormality of PNH, since patient material always consists of a mixture of both types of cells. However, a feasible approach was to study cloned lymphoblastoid cell lines (LCL) that displayed the PNH phenotype from PNH patients and to use as controls LCLs with normal phenotype obtained from the same patients. Comparative analysis of these two sets of cell lines revealed normal synthesis of phosphatidylinositol, but failure to incorporate mannose or NAcGlcN, thus pinpointing a block at the level of the NAcGlcN transfer.13
Molecular Genetics
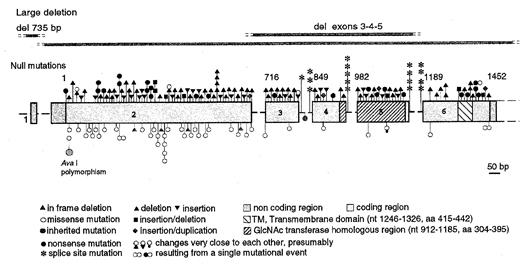
The PIG-A gene (so called for Phosphatidyl Inositol Glycan complementation group A) was isolated by expression cloning,14 i.e. through its ability to restore the expression of GPI-anchored proteins on the surface of cells lacking those proteins, including those from PNH patients.15 PIG-A maps to the short arm of the X chromosome on Xp22.1, within a region almost completely covered by physical contigs.16 It quickly became clear that acquired mutations of the PIG-A gene are responsible for PNH; to date, a total of 174 somatic mutations in the PIG-A gene (see Figure 4) have been identified by different investigators in more than 28 reports in 146 patients.17 Of these, (a) 135 are such that (large deletions, frameshifts, nonsense) we can predict complete functional inactivation of the PIG-A gene product (PIG-Ao); (b) 35 are missense mutations and 4 are small in frame deletions. These two groups presumably account for the existence in PNH patients of blood cells with complete deficiency of GPI-anchored proteins (PNH III) or partial deficiency of GPI-anchored proteins (PNH II). The two types not infrequently co-exist in the same patient, indicating that two different clones are present. PNH III red cells are naturally even more susceptible to C than PNH II red cells, and therefore the absolute and relative amounts of these and of the residual normal red cell population affect the rate of hemolysis considerably.
Structure and mutations of the PIG-A gene.
The coding region is shown in the form of boxes; introns are shown in the form of dashed lines (not drawn to scale). Nucleotide numbers are shown above the exons. Null mutations (frameshift, nonsense and splicing) are indicated above the exons. Missense mutations and in frame deletions are indicated below the exons. Each symbol represents one individual mutation. All mutations are somatic, except for the two shown in green. From reference 17 .
Structure and mutations of the PIG-A gene.
The coding region is shown in the form of boxes; introns are shown in the form of dashed lines (not drawn to scale). Nucleotide numbers are shown above the exons. Null mutations (frameshift, nonsense and splicing) are indicated above the exons. Missense mutations and in frame deletions are indicated below the exons. Each symbol represents one individual mutation. All mutations are somatic, except for the two shown in green. From reference 17 .
The predicted protein product of PIG-A consists of 484 amino acids. A hydrophobic region near the carboxyl terminus may be a transmembrane domain (amino acids 415-442). The hydrophilic carboxyl terminal region of 42 residues corresponds to the lumenal domain of PIG-A. Watanabe et al18 have shown that PIG-A, together with three other proteins (PIG-H, PIG-C and GPI1), constitutes a complex that mediates the first reaction step of the GPI anchor biosynthesis, the transfer of GlcNAc to phosphatidylinositol consistent with the biochemical studies mentioned above. The fact that PIG-A is part of an enzyme is also in keeping with the fact that PNH mutations are recessive.
PIG-A mutations can be demonstrated in the blood cells of a large majority of patients with PNH. Failure to find a mutation in the coding region of PIG-A in any individual patient is most likely due to technical reasons; however, it has not been formally excluded that in rare cases the PNH phenotype may arise through mutation of both alleles of an autosomal gene encoding any of the proteins required for the GPI biosynthetic pathway. The vast predominance of PIG-A mutations (over hypothetical mutations of such other genes) must be due to the fact that, because PIG-A is X-linked, an inactivating mutation (one-hit) will cause the PNH phenotype directly.
Thrombosis
Thrombosis is one of the most immediately life-threatening complications of PNH and yet one of the least understood in its pathogenesis. In principle, we can envisage three sorts of mechanisms: (a) Impaired fibrinolysis. The urokinase plasminogen activator receptor (uPAR) is a GPI-linked protein and is deficient in leukocytes belonging to the PNH clone.19 If leukocytes play a role in endogenous fibrinolysis, PNH leukocytes might be less efficient in this function due to their inability to bind urokinase. In addition, the levels of soluble u-PAR (which may normally help to regulate urokinase activity) are significantly increased in PNH patients.20 This could result in reduced endogenous fibrinolysis. However, tissue plasminogen activator is believed to be the main effector of the conversion of plasminogen to plasmin. (b) Hypercoagulability. The coagulation cascade, and therefore ultimately thrombin generation, may become activated in PNH. For instance, the C5b-9 complex has been shown to induce release of platelet micro-particles that express receptors for factor Va and exhibit prothrombinase21 and “tenase” (factor X cleavage) activity.22 Intravascular hemolysis also may release from red cells substances that have thromboplastin activity. However, in a series of 11 PNH patients, no abnormalities were seen in levels of thrombin/anti-thrombin complexes or in thrombin activation fragment F1.2.23 Thrombosis can occur in PNH patients even when they are fully anticoagulated with warfarin or hirudin, both of which would be expected to effectively prevent hypercoagulability through their effects on the common coagulation pathway. Therefore, activated coagulation itself is not likely to be the main culprit for thrombosis in PNH. (c) Hyperactivity of platelets. After treatment with C5b-9 PNH platelets undergo increased vesiculation and thrombin generation,24 and elevated levels of platelet derived micro-particles have been demonstrated in some PNH patients.25 The expression of activation markers is increased on the surface of platelets from PNH patients,23 probably because of abnormal C regulation, which may result in platelet activation.26,27 The lack of CD59 on the PNH platelet could lead to abnormal insertion of the C5b-9 complex in the platelet membrane, as is the case with the red cell.
Though these three factors may play a role in producing a thrombophilic state in PNH, it seems very likely that the primary cause lies in the PNH platelets, which are abnormal precisely because they belong to the PNH clone. This notion is supported by the occurrence of cerebral infarction in a patient who did not have PNH, but who had congenital CD59 deficiency and chronic hemolysis.28
Bone Marrow Failure
The close association between PNH and aplastic anemia (mentioned above) may reflect a shared pathogenetic basis.29 Aplastic anemia is essentially an organ-specific autoimmune disease. In the peripheral blood and bone marrow of patients with aplastic anemia it is possible to find increased numbers of `activated' CD8+ T-lymphocytes, which are able to inhibit the growth in vitro of both autologous and HLA-identical hematopoietic colonies.30 T-lymphocytes might be implicated in causing aplastic anemia in two ways. On one hand, by secreting interferon-γ and tumor necrosis factor-α they induce Fas expression on CD34+ cells. On the other hand, cytotoxic T-lymphocytes (CTLs) may ultimately cause apoptosis through Fas-FasL interaction (although this sequence of events has not yet been conclusively demonstrated), leading to depletion of HSC. The putative auto-antigens that incites and serves as a target of the autoreactive CTLs is not known. The strongest evidence for the immune basis of aplastic anemia is that the majority of patients respond to immunosuppressive treatment,31 Recently, skewing of the T cell repertoire has been demonstrated in a subset of patients with aplastic anemia by immunoscope analysis,32 and a detailed study conducted by the same approach has shown that one or several expanded T cells clones are present in PNH patients three times more frequently than in control subjects.33
Although PNH has elements of BMF, it is obviously different from aplastic anemia because of its other prominent clinical features (hemolysis and thrombosis). One possible way to look at the pathophysiology of PNH is that it results precisely from the co-existence of BMF with a large PIG-A mutant clone.34 In order to explain the co-existence of these two components, we can surmise in principle three possibilities: (i) The PNH clone and BMF co-exist by a sheer coincidence. (ii) The PNH clone causes BMF. (iii) BMF favors the development or the expansion of the PNH clone. Because AA and PNH are both rare diseases, the first possibility can be discarded on statistical grounds as being too improbable. The second possibility is unlikely, since PNH often develops in patients originally suffering from aplastic anemia, who therefore already had established BMF at a time when no PNH clone could be demonstrated. Thus, the third possibility seems the most likely, and it has recently obtained support through experimental findings in mice, as well as observations in humans.
Consequences of PIG-A Inactivation in Mice
When the PIG-A gene is targeted by homologous recombination in mouse embryonic stem (ES) cells, and these are then injected into blastocysts, viable chimeric animals can be obtained only if both the contribution to the embryo by the PIG-A null cells and the numbers of GPI-negative cells in their blood are low. Recently, by using the technique of conditional `knock-out' based on what is known in jargon as the cre-lox system, two groups have succeeded in producing mice that can be regarded as true models for human PNH.35, 36 Indeed, the mice have two discrete populations of red cells, granulocytes, monocytes, and lymphocytes: in first approximation, the flow cytometry patterns are remarkably similar to those seen in patients with PNH. In addition, although the mice are not anemic, they do have evidence of hemolysis and their red cells have increased susceptibility to complement (data on platelets and thrombosis are not yet available). Interestingly, the clinical course of the mice is very benign, and the proportion of PNH cells is remarkably stable, in spite of the fact that these mice are born with a large number of pre-formed PNH cells. In humans, by contrast, before development of the clinical picture of PNH, the PNH cell population must have undergone substantial expansion, since it arises from a single mutant stem cell.37 . It is tempting to surmise that the PNH cells in mice are very similar to PNH cells in human patients; but the mice do not have the human disease, PNH, because they do not have BMF. The fact that this is lacking supports the notion that the causation of BMF is independent of the PNH cell population itself.
PNH Clones and Microclones in Humans
We have already mentioned that PNH III and PNH II can co-exist in the same patient; indeed, by mutation analysis it is not infrequent to find two or more clones in the same patient. These findings are strongly suggestive of the hypothesis that these clones expand, simultaneously or in sequence, in response to a certain selective agent present in the patient's bone marrow environment.
On the other hand, is there evidence for PNH clones in normal people, and what is the fate of such clones? It has been shown recently that very small populations of PNH granulocytes and PNH erythrocytes are present in normal people, probably generated simply by the `background' level of somatic mutations in the PIG-A gene. Exactly as in patients with PNH, missense, frameshifts, and nonsense mutation have been identified. Two of these mutations had been found previously in patients with classical PNH, thus showing formally and conclusively that PIG-A gene mutations are not sufficient for the development of PNH.38 The fact that these very tiny PNH cell populations—microclones—do not expand clearly means that they do not have any intrinsic growth advantage and, conversely, that in PNH patients clones have expanded in a favorable environment.
Clinical Implications
Classification of PNH
How well do these concepts fit the clinical reality of patients with PNH? This can be tested by considering, in each patient, the relative roles of the PNH clone(s) and of BMF in determining the clinical picture (Table 4). At one end of the spectrum we may find a patient with a PNH clone so large that it masks BMF (almost) completely. The patient will have signs and symptoms of brisk hemolysis and may have serious thrombotic complications, but the peripheral blood count is normal or near normal; the patient can be said to have “florid PNH.” At the other end of the spectrum is a patient who meets all criteria for severe aplastic anemia and who is found (by sensitive analytical techniques) to have a very small proportion of GPI-negative cells in the blood. In such a patient the presence of the PNH clone is not likely to significantly affect the clinical course, and therefore we prefer to designate the patient as having “aplastic anemia with a PNH clone,” because it is the BMF rather than the PNH that must dictate therapeutic decisions. Intermediate situations are not uncommon. Most important, since there is a dynamic relationship between PNH clone(s) and BMF, transitions from one form to another may take place.
Therapy
It is clear from the above that in a patient with PNH we are dealing with two problems: the PNH clone and BMF (see Figure 5). The management of patients we have called “aplastic anemia with a PNH clone” does not differ from that of patients with straightforward aplastic anemia. By contrast, patients with “florid PNH” may present unique clinical problems, mainly massive hemolytic attacks and serious thrombotic complications. For these patients the two extreme choices are radical treatment by bone marrow transplantation (BMT) or supportive treatment only (of which red cell transfusion is the major component). When an HLA-identical sibling is available, allogeneic BMT is probably the treatment of choice for younger patients with moderate to severe cytopenias and, in view of the risk of life-threatening complications, must be regarded as an option to be offered to any young patient with PNH. By contrast, the past record of BMT from unrelated donors in PNH is poor, and it should still be regarded as experimental in the treatment of this condition. For patients who do not have an appropriate donor (and perhaps also for those who do), immunosuppressive treatment with ALG or ATG and cyclosporine A may be a good alternative. This type of treatment cannot be expected to eradicate the PNH clone because it aims instead to relieve the immune-mediated inhibition of normal hematopoiesis. However, in doing so it will limit if not eliminate the abnormal marrow environment in which the PNH clone thrives. Thus, for treating the BMF component of PNH we can capitalize on what has been learned from aplastic anemia. Confronting the consequences of having a large PNH clone (hemolysis and thrombosis) is a difficult challenge at the moment. Thrombosis, particularly in the abdomen, poses an immediate threat to the patient. Unfortunately even well-managed anticoagulant treatment does not always prevent thrombosis, although in some cases thrombolytic therapy can resolve it.39 As for intravascular hemolysis, despite the impressive advances of complement research we still do not have a clinically applicable method to inhibit the effector pathway—there is a dire need to develop new ideas in this area.
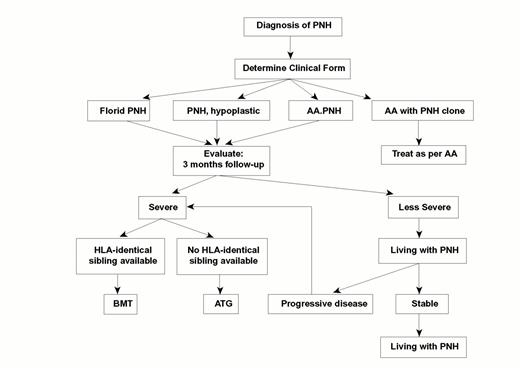
Guidelines for the management of PNH.
This algorithm40 is based on the consideration that patients with this condition vary considerably (a) in terms of clinical severity, and (b) in terms of the contributions of the PNH clone and of bone marrow failure (BMF) respectively to determining the overall clinical picture. Some patients have been cured by bone marrow transplantation (BMT); at the other end of the spectrum, some of the patients who for a long time have been `living with PNH'—by choice or otherwise—have eventually experienced spontaneous recovery.4
Guidelines for the management of PNH.
This algorithm40 is based on the consideration that patients with this condition vary considerably (a) in terms of clinical severity, and (b) in terms of the contributions of the PNH clone and of bone marrow failure (BMF) respectively to determining the overall clinical picture. Some patients have been cured by bone marrow transplantation (BMT); at the other end of the spectrum, some of the patients who for a long time have been `living with PNH'—by choice or otherwise—have eventually experienced spontaneous recovery.4
Conclusion: A Coherent Model for the Pathogenesis of PNH
In terms of the nosologic classification of human diseases, PNH is rather special and perhaps unique for at least two reasons: (a) Although it results from somatic mutations, it is not a malignant disease. (b) It is probably the only acquired hemolytic anemia that is due to an intrinsic red cell abnormality.
On the basis of current knowledge, we can formulate a model for the pathogenesis of PNH (Figure 6) which explains at least most of its clinical and hematological features, as follows:
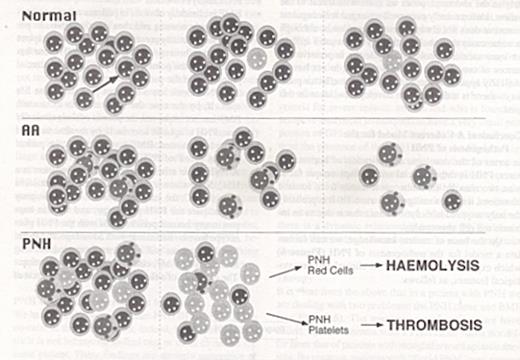
The role of somatic mutation and auto-immune mediated bone marrow failure in the pathophysiology of PNH.
The top panel is a cartoon of normal hematopoietic stem cells (HSC): the arrow indicates a somatic mutation in the PIG-A gene in one of the HSC. As a result, the cell and its progeny lose surface GPI-anchored proteins. As time goes on, micro clones arising from such mutant cells may become exhausted, and new ones may arise: however, there is no clonal expansion. The middle panel illustrates the presence in the bone marrow of auto reactive immune cells, which may be cytotoxic T cells: these attack the HSC, which gradually decrease in numbers, eventually resulting in the picture of aplastic anemia (AA). The bottom panel illustrates the consequences of the co-existence of a PIG-A somatic mutation and autoreactive immune cells in the bone marrow. If we make the specific hypothesis that the target of the autoimmune attack is a GPI-anchored protein, the mutant clone will expand as a result of negative selection against the normal HSC. As a result, the majority of hematopoiesis will consist of GPI-anchored protein deficient cells. The large numbers of c-susceptible red cells will cause hemolytic anemia; the large numbers of abnormal platelets will cause a high risk of thrombotic complications.
The role of somatic mutation and auto-immune mediated bone marrow failure in the pathophysiology of PNH.
The top panel is a cartoon of normal hematopoietic stem cells (HSC): the arrow indicates a somatic mutation in the PIG-A gene in one of the HSC. As a result, the cell and its progeny lose surface GPI-anchored proteins. As time goes on, micro clones arising from such mutant cells may become exhausted, and new ones may arise: however, there is no clonal expansion. The middle panel illustrates the presence in the bone marrow of auto reactive immune cells, which may be cytotoxic T cells: these attack the HSC, which gradually decrease in numbers, eventually resulting in the picture of aplastic anemia (AA). The bottom panel illustrates the consequences of the co-existence of a PIG-A somatic mutation and autoreactive immune cells in the bone marrow. If we make the specific hypothesis that the target of the autoimmune attack is a GPI-anchored protein, the mutant clone will expand as a result of negative selection against the normal HSC. As a result, the majority of hematopoiesis will consist of GPI-anchored protein deficient cells. The large numbers of c-susceptible red cells will cause hemolytic anemia; the large numbers of abnormal platelets will cause a high risk of thrombotic complications.
PNH always co-exists with BMF.
BMF is clinically obvious in patients who first manifest aplastic anemia and then develop PNH. In patients who initially present with PNH, BMF may not be obvious, because at diagnosis the PNH clone has expanded to the point where it provides a substantial proportion of the patient's hematopoiesis.
The PNH clone has a long but probably finite life span. If, by the time the PNH clone is exhausted, BMF has not improved, the patient evolves clinically from PNH to aplastic anemia. If, by the time the PNH clone is exhausted, BMF has improved, the patient will be `cured' of PNH.
A PNH clone arises through a PIG-A mutation in a HSC. Since there is only one active X-chromosome in each HSC, the mutated stem cell and its progeny will acquire the PNH phenotype, and this can happen in any normal person. Cells with the PNH phenotype have no intrinsic growth advantage, and therefore PNH clones will not normally expand. As long as there is no BMF, clinical PNH will not develop.
The existence of a florid PNH clone while the rest of hematopoiesis is depressed suggests that the PNH clone can be spared selectively from the injury affecting the rest of the bone marrow.
In order to explain the previous statement, we may surmise specifically that the damage to stem cells causing BMF is mediated through a GPI-linked surface molecule: in this case, the PNH cells lacking these molecules will survive.
Thus, the very defect of the PNH clone may endow it with a relative survival or growth advantage in a patient with BMF. If the patient has such a clone, he or she will present with PNH; otherwise he or she would present with overt aplastic anemia. Thus, the development of PNH is conditional on a background of BMF.
(8) A large PNH clone carries with it intravascular hemolysis and thrombosis, the `classical' manifestations of PNH.
I thank all my colleagues with whom I have been fortunate to work on PNH over the years, and I am very grateful to D. Araten, A. Karadimitris, R. Notaro and D. Nafa for help with this paper.