Abstract
This review focuses on antithrombotic therapy for venous thromboembolism and covers a diverse range of topics including a discussion of emerging anticoagulant drugs, a renewed focus on thrombolytic agents for selected patients, and an analysis of the factors leading to adverse events in patients on warfarin, and how to optimize therapy. In Section I Dr. Weitz discusses new anticoagulant drugs focusing on those that are in the advanced stages of development. These will include drugs that (a) target factor VIIa/tissue factor, including tissue factor pathway inhibitor and NAPc2; (b) block factor Xa, including the synthetic pentasaccharide and DX9065a; (c) inhibit factors Va and VIIIa, i.e., activated protein C; and (d) block thrombin, including hirudin, argatroban, bivalirudin and H376/95. Oral formulations of heparin will also be reviewed.
In Section II, Dr. Comerota will discuss the use of thrombolysis for selected patients with venous thromboembolism. Fibrinolytic therapy, which has suffered from a high risk/benefit ratio for routine deep venous thrombosis, may have an important role to play in patients with iliofemoral venous thrombosis. Dr. Comerota presents his own results with catheter-directed thrombolytic therapy and the results from a large national registry showing long-term outcomes and the impact on quality of life.
In Section III, Dr. Ansell presents a critical analysis of the factors responsible for adverse events with oral anticoagulants and the optimum means of improving outcomes. The poor status of present day anticoagulant management is reviewed and the importance of achieving a high rate of “time in therapeutic range,” is emphasized. Models of care to optimize outcomes are described, with an emphasis on models that utilize patient self-testing and patient self-management of oral anticoagulation which are considered to be the ultimate in anticoagulation care. The treatment of venous and arterial thromboembolism is undergoing rapid change with respect to the development of new antithrombotic agents, an expanding list of new indications, and new methods of drug delivery and management. In spite of these changes, many of the traditional therapeutics are still with us and continue to play a vital role in the treatment of thromboembolic disease. The following discussion touches on a wide range of therapeutic interventions, from old to new, exploring the status of anticoagulant drug development, describing a new intervention for iliofemoral venous thrombosis, and analyzing the critical factors for safe and effective therapy with oral anticoagulants.
I. New Anticoagulant Drugs
Jeffrey I. Weitz, M.D.*
Hamilton Civic Hospitals Research Center, 711 Concession Street, Hamilton, Ontario L8V 1C3, Canada
Thrombogenesis
Arterial thrombosis is the most common cause of myocardial infarction, stroke and limb gangrene. Because arterial thrombi, which form under conditions of high flow, consist of platelet aggregates held together by small amounts of fibrin, strategies to inhibit arterial thrombogenesis focus mainly on drugs that block platelet function, but often include anticoagulants to prevent fibrin deposition.1 In contrast, venous thrombi, which can cause post-phlebitic syndrome or lead to pulmonary embolism and death, consist mainly of fibrin and red blood cells, and develop under low flow conditions in the deep calf veins. Coagulation at these sites is initiated by vascular trauma and is augmented by venous stasis. Anticoagulants are the drugs of choice for the prevention or treatment of venous thrombosis, or of cardioembolic events.
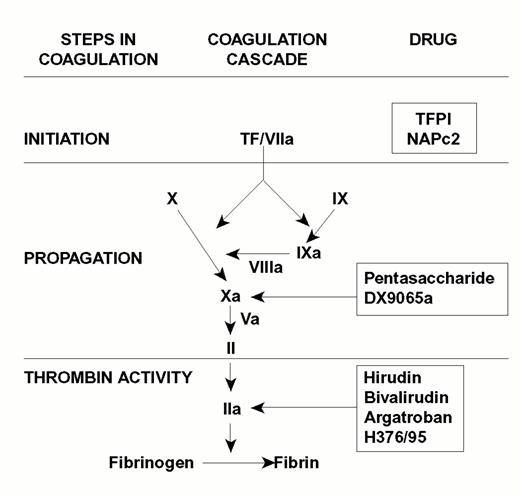
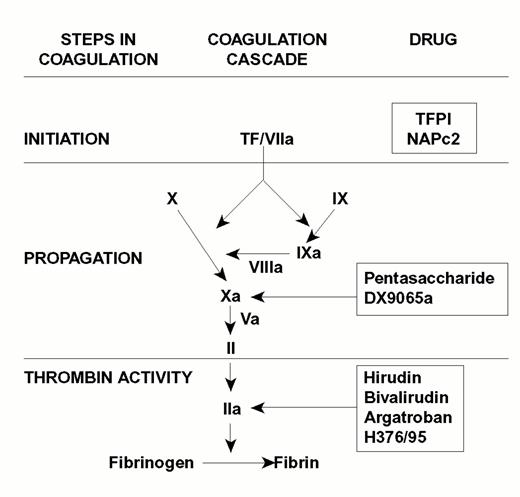
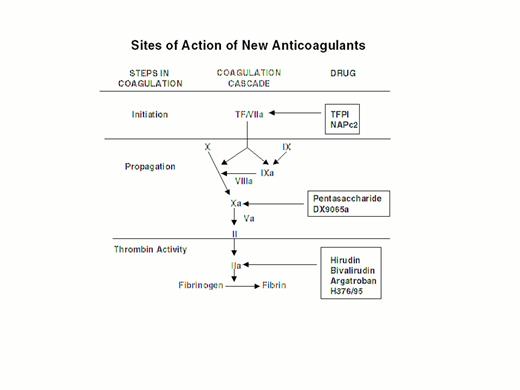
Injury to the arterial or venous wall exposes nonvascular tissue factor-expressing cells to blood.2 Exposed tissue factor triggers coagulation (Figure 1) by binding factor VII or activated factor VII (factor VIIa). Tissue factor-bound factor VIIa undergoes autoactivation,3 and the resultant factor VIIa/tissue factor complex then activates factor IX and factor X, leading to the generation of factor IXa and factor Xa, respectively. Factor IXa binds to factor VIIIa on membrane surfaces to form intrinsic tenase, the complex that activates factor X. By feedback activation of factor VII, factor Xa amplifies the initiation of clotting.2
Steps in blood coagulation and sites of action of new anticoagulants.
Initiation of coagulation is triggered by the factor VIIa/tissue factor complex (VIIa/TF), which activates factor IX (IX) and factor X (X). Activated factor IX (IXa) propagates coagulation by activating factor X in a reaction that utilizes activated factor VIII (VIIIa) as a cofactor. Activated factor X (Xa), with activated factor V (Va) as a cofactor, converts prothrombin (II) to thrombin (IIa). Thrombin then converts fibrinogen to fibrin. Tissue factor pathway inhibitor (TFPI) and nematode anticoagulant peptide (NAPc2) target VIIa/TF, whereas synthetic pentasaccharide and DX-9065a inactivate Xa. Hirudin, bivalirudin, argatroban, and H376/95 inactivate thrombin.
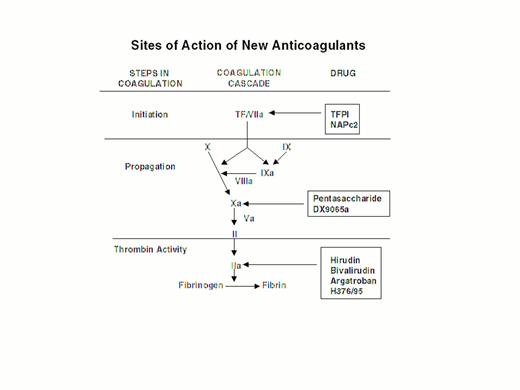
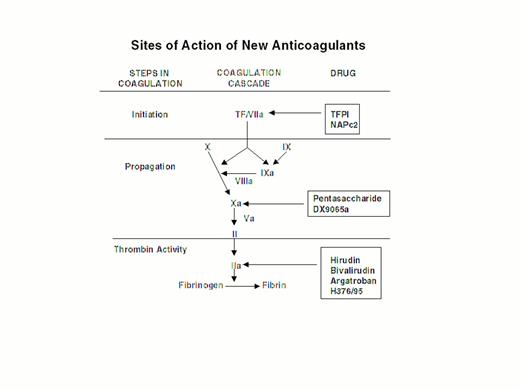
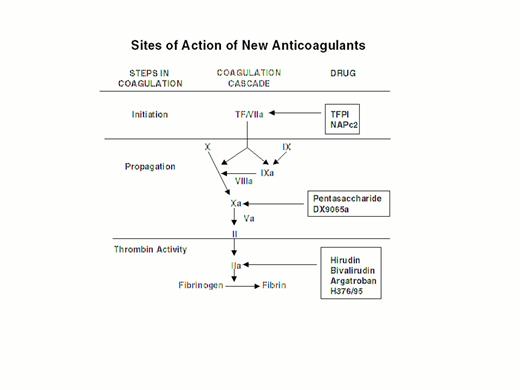
Steps in blood coagulation and sites of action of new anticoagulants.
Initiation of coagulation is triggered by the factor VIIa/tissue factor complex (VIIa/TF), which activates factor IX (IX) and factor X (X). Activated factor IX (IXa) propagates coagulation by activating factor X in a reaction that utilizes activated factor VIII (VIIIa) as a cofactor. Activated factor X (Xa), with activated factor V (Va) as a cofactor, converts prothrombin (II) to thrombin (IIa). Thrombin then converts fibrinogen to fibrin. Tissue factor pathway inhibitor (TFPI) and nematode anticoagulant peptide (NAPc2) target VIIa/TF, whereas synthetic pentasaccharide and DX-9065a inactivate Xa. Hirudin, bivalirudin, argatroban, and H376/95 inactivate thrombin.
Factor Xa propagates coagulation by binding to factor Va on membrane surfaces to form the prothrombinase complex. Factor Xa within this complex activates prothrombin to thrombin which then dissociates from the membrane surface and converts fibrinogen to fibrin monomer. Fibrin monomers polymerize to form the fibrin mesh, which is stabilized and crosslinked by factor XIIIa, a thrombin-activated transglutaminase. Thrombin amplifies its own generation by feedback activation of factor V and factor VIII. Thrombin also can activate factor XI, thereby leading to further factor Xa generation.4
New Anticoagulant Strategies
Anticoagulant strategies to inhibit thrombogenesis have focused on inhibiting thrombin, preventing thrombin generation, or blocking initiation of coagulation (Figure 1). Coagulation factors that have been targeted for inactivation include thrombin, factor Xa, factor IXa, and the factor VIIa/tissue factor complex. Other approaches to attenuating thrombogenesis include enhancing endogenous anticoagulant pathways or promoting fibrinolysis. Only a small number of potential candidate drugs are under development, and even fewer have progressed to clinical testing. Only compounds in more advanced stages of clinical development, which are listed in Table 1, will be discussed here; new fibrinolytic drugs and antiplatelet agents will not be described.
Thrombin Inhibitors
Thrombin can be inhibited indirectly by agents that activate naturally occurring thrombin inhibitors (namely, antithrombin or heparin cofactor II) or directly by drugs that bind to thrombin and prevent its interaction with substrates.
Indirect Thrombin Inhibitors
Unfractionated heparin (UFH) and low-molecular-weight heparin (LMWH) are cornerstones for prevention and treatment of venous thrombosis and also are widely used in combination with antiplatelet drugs or thrombolytic agents for treatment of acute coronary ischemic syndromes. Because LMWH can be given without laboratory monitoring, it is a useful drug for out-of-hospital treatment.5 Consequently, LMWH is gradually replacing heparin for treatment of venous thrombosis. LMWH also is useful for treatment of unstable angina.5
Delivery systems have recently been developed making it possible to give UFH or LMWH orally. These delivery systems utilize synthetic amino acids such as sodium N-(8[2-hydroxybenzoyl]amino) caprylate (SNAC) to facilitate heparin absorption by the gut.6 Although absorption is limited and somewhat variable, sufficient amounts of heparin can be delivered orally to prolong the activated partial thromboplastin time.6 With phase I7 and phase II8 trials completed, phase III studies comparing SNAC/heparin with LMWH for thromboprophylaxis in patients undergoing elective hip or knee arthroplasty are now underway.
Dermatan sulfate, which acts as an anticoagulant by activating heparin cofactor II,9 has been shown to be more effective than low-dose UFH for thromboprophylaxis in cancer patients.10 Because its low specific activity and poor solubility limit the amount of drug that can be given by subcutaneous injection, dermatan sulfate has yet to be evaluated in the treatment setting.
Direct Thrombin Inhibitors
Direct thrombin inhibitors have potential advantages over UFH because they can inhibit fibrin-bound thrombin,11,12,13 an important mediator of thrombus growth. In addition, direct thrombin inhibitors produce a more predictable anticoagulant response than UFH because they do not bind to plasma proteins and are not neutralized by platelet factor 4, a heparin-binding protein released from activated platelets.14 Hirudin, bivalirudin and argatroban are parenteral direct thrombin inhibitors that are approved for certain indications, whereas H376/95 is an orally active agent currently under investigation.
Hirudin
Hirudin, a 65-amino acid polypeptide originally isolated from the salivary glands of the medicinal leech Hirudo medicinalis, is now available through recombinant DNA technology.15 It is a potent and specific inhibitor of thrombin that forms a stoichiometric, slowly reversible complex with the enzyme.16 Its globular amino-terminal domain interacts with the active site of thrombin, while its carboxy-terminal domain binds to exosite 1 on the enzyme.17
Hirudin is cleared predominantly by the kidneys, undergoes little hepatic metabolism18 and has a plasma half-life of 40 minutes after intravenous administration and approximately 120 minutes after subcutaneous injection. Its almost irreversible binding to thrombin may be considered a potential weakness, as no antidote is available should bleeding occur.
Hirudin has been used successfully to treat patients with thrombotic complications of heparin-induced thrombocytopenia (HIT)19,20 and has been licensed for this indication in North America.21 It also has been used effectively as an alternative to heparin during cardiopulmonary bypass in a small number of patients with HIT.22,23 Hirudin has been shown to be superior to low-dose subcutaneous UFH or LMWH for thromboprophylaxis in patients undergoing elective hip arthroplasty and does not increase the risk of bleeding in this high-risk setting.24,25 In patients with unstable angina and non-ST-elevation myocardial infarction, hirudin appears to be more effective than UFH.26,27 Although hirudin increases the risk of major bleeding in these patients, there is no increase in life-threatening bleeds.27 Hirudin is now under consideration for licensing in patients with unstable angina and non-ST segment elevation myocardial infarction.
Bivalirudin
A semi-synthetic bivalent thrombin inhibitor, bivalirudin is comprised of a dodecapeptide analogue of the carboxyterminal of hirudin that binds to exosite 1 on thrombin linked to an active-site-directed moiety, D-Phe-Pro-Arg-Pro, by four glycine residues.28 Unlike hirudin, bivalirudin produces only transient inhibition of the active site of thrombin because once bound to thrombin, the Arg-Pro bond on the amino-terminal extension of bivalirudin is cleaved, converting bivalirudin into a lower affinity inhibitor.29 The shorter half-life of bivalirudin may render bivalirudin safer than hirudin. Based on phase III trials showing enhanced safety of bivalirudin relative to UFH in patients undergoing coronary angioplasty,30,31 bivalirudin is now licensed in North America for this indication. In contrast to hirudin, only a fraction of bivalirudin is renally excreted, suggesting that hepatic metabolism and proteolysis at other sites contribute to its clearance.32
Argatroban
A carboxylic acid derivative, argatroban binds noncovalently to the active site of thrombin.33 Argatroban is an effective alternative to heparin in patients with HIT and is approved for this indication. Studies are now underway to evaluate argatroban for treatment of arterial thrombosis.
H376/95
A prodrug oral formulation of melagatran, H376/95 is an uncharged lipophilic drug with little intrinsic activity against thrombin. H376/95 is well absorbed from the gastrointestinal tract and undergoes rapid biotransformation to melagatran, an active site-directed thrombin inhibitor.34,35 The drug produces a predictable anticoagulant response after oral administration, and little or no laboratory monitoring appears to be necessary. Phase II studies with H376/95 for prevention and treatment of venous thrombosis have been completed, and phase III trials for these indications are now underway.
Factor IXa Inhibitors
Strategies to block factor IXa, a factor essential for amplification of coagulation, include active-site-blocked factor IXa and monoclonal antibodies against factor IX/IXa.
Active-site-blocked factor IXa
By competing with factor IXa for incorporation into the intrinsic tenase complex that assembles on the surface of the activated platelets, active site-blocked factor IXa inhibits clot formation in vitro and blocks coronary artery thrombosis in a canine model.36 These observations support the concept that agents that inhibit factor IXa will modulate thrombosis.
Antibodies against factor IX/IXa
Monoclonal antibodies against factor IX/IXa either block factor X activation by factor IXa37 or bind to the Gla-domains of factor IX and inhibit factor IX activation in addition to blocking factor IXa activity.38,39 A chimeric humanized derivative of the latter antibody has antithrombotic activity in a rat arterial thrombosis model.38,39 Phase I studies with this agent have been completed.
Factor Xa Inhibitors
Factor Xa inhibitors include a synthetic pentasaccharide, an analogue of the pentasaccharide sequence of heparin40 that acts indirectly by activating antithrombin, as well as direct inhibitors that include recombinant analogues of natural inhibitors and synthetic drugs that target the active site of factor Xa. In contrast to UFH and LMWH, which have limited ability to inhibit platelet-bound factor Xa,5,42 direct inhibitors of factor Xa inactivate both factor Xa bound to phospholipid surfaces and free factor Xa.41
Synthetic Pentasaccharide
Synthetic pentasaccharide, which has higher specific activity than UFH or LMWH,40 enhances the rate of factor Xa inactivation by antithrombin but has no effect on the rate of thrombin inhibition. The drug is given subcutaneously on a once-daily basis. Phase III trials comparing synthetic pentasaccharide with LMWH for venous thrombosis prevention and treatment are well underway.
Natural inhibitors
Natural inhibitors of factor Xa include tick anticoagulant peptide (TAP), antistasin and lefaxin, none of which has been tested in humans. TAP, a 60-amino acid polypeptide isolated from the soft tick, Ornithodoros moubata, forms a stoichiometric complex with factor Xa.41 TAP appears to bind to factor Xa in a two-step fashion.41 An initial low affinity interaction involves a site distinct from the catalytic site of the enzymes, which may be analogous to exosite 1 of thrombin. This is followed by a high affinity interaction with the active site, which results in formation of a stable enzyme-inhibitor complex.
Antistasin is a 119 amino acid polypeptide isolated from the salivary glands of the Mexican leech, Haementeria officinalis.43 Both native and recombinant forms of antistasin are tight-binding, slowly reversible inhibitors of factor Xa.44 Like TAP, antistasin is highly selective for factor Xa. Lefaxin is a 30 kDa polypeptide isolated from the saliva of the Haemanteria depressa leech.45 Although the gene encoding this protein has yet to be cloned, limited sequence analysis shows no homology between lefaxin and other natural inhibitors of factor Xa.
Synthetic factor Xa inhibitors
Nonpeptidic, low-molecular-weight, reversible inhibitors of factor Xa, such as DX-9065a,46 YM-60828,47 SF 303 and SK 549,48,49 are effective in animal thrombosis models. Intravenous DX9065a is currently undergoing phase II testing in patients with unstable angina. YM-60828, a more potent analog of DX-9065a, has been reported to have oral bioavailability in squirrel monkeys,47 whereas SK 549 exhibits oral bioavailability in rabbits.48,49
Factor VIIa/Tissue Factor Pathway Inhibitors
Strategies aimed at blocking the factor VIIa/tissue factor pathway have received much recent attention given that coagulation is initiated via this pathway.2 Recombinant tissue factor pathway inhibitor (TFPI) and recombinant nematode anticoagulant peptide (NAPc2) are the agents in the most advanced stages of development.
TFPI
Only small amounts of this factor Xa-dependent inhibitor of factor VIIa circulate in blood in the free state or are stored in platelets. Most of the TFPI is bound to lipoproteins or to the endothelium.50 Full-length TFPI is released from the endothelium when UFH or LMWH is given, presumably because these agents displace TFPI bound to endothelial glycosaminoglycans. When administered intravenously, TFPI has a short half-life because it is rapidly cleaved into non-functional truncated forms by an unknown protease. In pigs, TFPI attenuates injury-induced neointimal hyperplasia, and it inhibits smooth muscle cell migration in vitro. TFPI attenuates the coagulopathy and improves survival in sepsis models in baboons or rabbits.50 Based on these results, TFPI is now undergoing phase III evaluation in patients with sepsis.
NAPc2
Small proteins have been isolated from Ancylostoma caninum that contain Ascaris-type protease motifs.51 Some of these proteins directly inhibit factor Xa, whereas others, like NAPc2, bind to a noncatalytic site on factor X or factor Xa and inhibit factor VIIa within the factor VIIa/tissue factor complex.52 Because it binds to factor X, as well as factor Xa, NAPc2 has a half-life of almost 50 hours after subcutaneous injection. Functionally, however, NAPc2 behaves like TFPI and attenuates sepsis-induced coagulopathy in laboratory animals. NAPc2 is currently undergoing phase II testing for prevention of venous thrombosis in patients undergoing elective knee arthroplasty.
Enhancement of Endogenous Anticoagulant Activity
Strategies aimed at enhancing endogenous anticoagulant activity have focused on the protein C anticoagulant pathway and include administration of (a) protein C or activated protein C concentrates, (b) soluble thrombomodulin, (c) thrombin derivatives that preferentially activate protein C, or (d) small molecules that bind to thrombin and induce allosteric changes similar to those evoked by thrombin's interaction with thrombomodulin.
Protein C derivatives
Both plasma-derived and recombinant forms of protein C are available. Intravenous activated protein C shows promise in the treatment of patients with sepsis-induced coagulopathy,53 and is currently undergoing phase III testing for this indication.
Soluble thrombomodulin
Soluble thrombomodulin complexes with thrombin and induces a conformational change in the active site of the enzyme that abolishes its procoagulant activity and converts it into a potent activator of protein C.54 Now available by recombinant DNA technology,55 soluble thrombomodulin is an effective antithrombotic agent in a variety of animal models.56,57
Thrombin variants
Thrombin can be mutated to dissociate its procoagulant and anticoagulant substrate specificity, and when infused into animals it can activate protein C and have minimal procoagulant activity. Most promising of the thrombin variants are those that have the Glu residue at position 229 replaced by an Ala or Lys residue.58,59
Allosteric modulators of thrombin
A promising strategy is the development of small molecules that bind to thrombin and induce conformational changes in its active site similar to those evoked by thrombin's interaction with thrombomodulin. Small ligands of limited potency have recently been identified.60
Modulation of Endogenous Fibrinolytic Activity
Although traditional antithrombotic strategies have been aimed at inhibiting platelet function or blocking coagulation, a better understanding of physiologic fibrinolysis has identified potential methods to enhance endogenous fibrinolytic activity. These include (a) blocking type 1 plasminogen activator inhibitor (PAI-1), (b) inhibition of carboxypeptidase B-like enzymes, or (c) inhibition of activated factor XIII (factor XIIIa).
PAI-1 inhibitors
PAI-1 is the major physiologic inhibitor of tissue-type plasminogen activator (t-PA) and urinary-type plasminogen activator (u-PA). Consequently, inhibition of PAI-1 results in increased endogenous fibrinolytic activity. PAI-1 activity can be reduced by decreasing PAI-1 gene expression or by reducing the activity of PAI-1. Lipid-lowering drugs, such as niacin and fibrates, decrease PAI-1 synthesis in vitro.61,62 These agents are not specific for PAI-1, however, and also affect the synthesis of other proteins.
Peptides have been identified that block PAI-1 activity either by preventing insertion of the reactive center loop upon cleavage by the target protease63 or by converting PAI-1 into a latent conformation.64 However, the effectiveness of these agents has yet to be tested in vivo. A more promising strategy is the development of small molecule PAI-1 inhibitors, some of which exhibit antithrombotic activity in vivo.65
Procarboxypeptidase B inhibitors
Procarboxypeptidase B or thrombin-activatable fibrinolysis inhibitor (TAFI) is a latent carboxypeptidase B-like enzyme that is activated by the thrombin/thrombomodulin complex.66 Upon activation, the carboxypeptidase attenuates fibrinolysis, presumably by cleaving carboxy-terminal lysine residues from fibrin.67 Removal of these lysine residues decreases plasminogen or plasmin binding to fibrin, thereby retarding the lytic process. Given this mechanism of action, inhibitors of procarboxypeptidase B should enhance fibrinolytic activity, a concept supported by studies in dogs and rabbits demonstrating that a potato-derived carboxypeptidase B inhibitor increases t-PA-induced thrombolysis.68,69
Recently, thrombin variants have been identified that have decreased ability to activate procarboxypeptidase B yet retain their ability to activate protein C.70 These findings raise the possibility that the antifibrinolytic activity of thrombin can be blocked without affecting its ability to activate the protein C pathway.
Factor XIIIa inhibitors
A thrombin-activated transglutaminase, factor XIIIa crosslinks the α- and γ-chains of fibrinogen to form α-polymers and γ-dimers, respectively. Crosslinking stabilizes the fibrin polymer and renders it more refractory to degradation by plasmin.71 Inhibition of factor XIIIa, therefore, has the potential to increase the susceptibility of the thrombus to lysis. Tridegin, a peptide isolated from the giant Amazon leech, Haementeria ghilianti, is a specific inhibitor of factor XIIIa and enhances fibrinolysis in vitro when added prior to clotting of fibrinogen.72,73 Destabilase, a leech enzyme that hydrolyzes γ-γ crosslinks,74,75 also provides a promising approach to reversing the consequences of factor XIIIa-mediated fibrin crosslinking.
Challenges and Opportunities for New Anticoagulant Drugs
Further clinical testing is needed to define the role of new anticoagulants and to establish their benefit-to-risk profiles and their cost-effectiveness, especially when evaluating drugs with marginal advantages over existing agents. Although hirudin has been shown to be superior to low-dose UFH or LMWH for thromboprophylaxis in patients undergoing major orthopedic surgery of the lower limbs,24,25 hirudin is unlikely to gain wide acceptance for this indication unless its cost is comparable to that of LMWH. Only limited information is available on the use of hirudin for the treatment of venous thrombosis. Cost considerations may also limit the utility of synthetic pentasaccharide40 or NAPc252 in this setting, should these drugs prove superior to those in current use.
The success of hirudin for thromboprophylaxis in high-risk patients bodes well for orally available agents in this class. With progressive reductions in hospital stay, and evidence that the risk of thrombosis remains high for several weeks after major orthopedic surgery to the lower limbs,76,77,78 orally available drugs that have a rapid onset of action and need little or no laboratory monitoring may prove to be more convenient than LMWH or coumarin derivatives. Although oral delivery systems for UFH or LMWH are promising,7,8 variable absorption may limit the utility of this approach. In contrast, a prodrug form of melagatran exhibits good bioavailability after oral administration34,35 and has undergone promising phase II testing for thromboprophylaxis in orthopedic patients.
The effectiveness of orally available drugs that target factor Xa, or clotting enzymes higher in the coagulation cascade, remains to be established. However, the success of synthetic pentasaccharide in phase II thromboprophylaxis trials suggests that factor Xa inhibitors will be effective in this setting.
There remains a need for orally active anticoagulants that are safer than coumarin derivatives given the mounting evidence that patients who develop venous thromboembolism in the absence of identifiable risk factors require long-term anticoagulation.79,80,81,82 Orally active drugs that target thrombin or factor Xa have the potential to be superior to coumarins for this indication, if they can be administered safely with little or no laboratory monitoring.
II. Thrombolytic Therapy for Iliofemoral Deep Venous Thrombosis: An Opportunity Missed?
Anthony J. Comerota, M.D., FACS*
Temple University, Vascular Surgery, 3401 N. Broad Street, Parkinson Pavilion Bldg., Suite 400, Philadelphia PA 19140
Patients with the post-thrombotic syndrome are frequently encountered in clinical practice, but therapeutic interventions are often of little help. Venous hypertension and valvular incompetence are important pathophysiologic precursors of the post-thrombotic syndrome and occur frequently following iliofemoral venous thrombosis. Åkesson et al83 followed 20 patients with iliofemoral venous thrombosis who were treated with conventional anticoagulation. After 5 years, 95% of the patients had ambulatory venous hypertension and 90% demonstrated objective signs and symptoms of venous insufficiency. Fifty percent of patients had calf muscle pump dysfunction, 15% suffered the debilitating symptoms of venous claudication and another 15% developed venous ulcers during the short follow-up. O'Donnell and Browse reported a 10-year follow-up of patients with iliofemoral venous thrombosis.84 They objectively documented the extreme post-thrombotic morbidity of this condition, with many patients finding it difficult to maintain their employment due to incapacitating swelling and leg ulceration. Although restoring patency to the deep venous system is an ideal goal of therapy for acute deep venous thrombosis (DVT), pharmacologic and mechanical methods designed to clear the deep venous system remain controversial. Accumulated data85 (Table 2) and randomized trials86,87 (Table 3) demonstrate that more patients have patency of their venous system or reduced post-thrombotic symptoms after treatment with thrombolytic therapy compared to standard anticoagulation, yet thrombolytic agents are used infrequently by most physicians in the United States.
Since standard anticoagulant therapy has failed to reduce the post-thrombotic syndrome and systemic lytic therapy has been disappointing, we employed catheter-directed thrombolysis for patients with no contraindications to lytic therapy, and iliofemoral venous thrombectomy for those with major contraindications or who fail catheter-directed thrombolysis88 (Figures2 and 3). The following reviews our results of catheter-directed thrombolysis for iliofemoral DVT and places these results into perspective with those of other centers that have adopted a similar approach.
Catheter-Directed Thrombolysis
Between 1988 and 2000, 54 patients were treated with catheter-directed thrombolysis for occlusive iliofemoral DVT at Temple University Hospital. Mean age was 45 years (range 17-68 years), and average duration of leg symptoms was 5.2 days (range 2-30 days). All patients had the diagnosis established with venous duplex imaging and iliofemoral phlebography. Five patients were known to have recent pulmonary emboli (PE), and the remainder had no symptoms of PE. Seven of our patients had vena caval filters placed at the time of treatment. Three patients had vena caval thrombosis extending above a previously placed vena caval filter, and four patients had a vena caval filter inserted as part of their current therapy.
In patients in whom the catheter-directed approach failed and those with an absolute contraindication to thrombolytic therapy or multiple relative contraindications, a venous thrombectomy and adjunctive arteriovenous fistula was performed under general anesthesia. Patient follow-up was 2-98 months, with a mean of 46 months.
Initially, direct intra clot infusion was achieved by advancing a catheter from the contralateral femoral vein, the right internal jugular vein or both to the site of the clot. More recently, an ipsilateral popliteal vein puncture was the preferred approach; ultrasound guided posterior tibial vein puncture was used in selected patients. Urokinase was used initially, delivering a 250,000-500,000 U bolus followed by a continuous infusion of 250,000-300,000 U/hr. With the recent removal of urokinase from the market because of concerns of potential viral contamination, seven recent patients were treated with recombinant tissue plasminogen activator (rt-PA). A bolus of 4-8 mg of rt-PA was given followed by 2-4 mg/hr. All patients received heparin at 500-1,000 u/hr. Venous duplex imaging was used to follow lysis of the infrainguinal clot and as much of the iliac venous system as could be visualized. Therapy continued until maximal lysis was achieved. Following completion of lytic therapy, heparin was continued and the patient converted to oral anticoagulation. If the infusion catheter could not be appropriately positioned within the iliac vein thrombus, a venous thrombectomy was recommended.
Table 4 summarizes the outcome of catheter-directed thrombolysis. Ninety-four percent of patients had successful positioning of the catheter and 88% had a successful lytic outcome. Of those who failed, two had known chronic occlusions and in two patients the reason for failure was unknown. Two patients suffered early rethrombosis due to persistent iliac vein stenosis.
Urokinase was the lytic agent used in 47 of the 54 patients. The duration of urokinase infusion ranged from 12-72 hours with the mean of 30 hours and an average total dose of 7.8 million units. Of the seven patients who received rt-PA, the duration of therapy was 12-40 hours with a mean of 22 hours and an average total dose of 48 mg.
The most common complication, puncture site hematoma, occurred in 15% of our patients. Blood transfusion was required in four patients (7%). One patient required operative evacuation of a puncture site hematoma and repair of the common femoral vein. One patient suffered perforation of the common femoral vein at the origin of the profunda during initial catheter positioning and was not infused with a lytic agent. Five patients who had unsuccessful thrombolysis were subsequently treated with operative venous thrombectomy.
The long-term results of treatment are categorized according to their clinical outcome and the SVS/ISCVS clinical classification89 of chronic venous disease.
The results of catheter-directed thrombolysis for acute iliofemoral DVT have been gratifying when contrasted to the natural history of patients treated with standard anticoagulation. Plate and colleagues demonstrated that patients who had patency restored to their thrombosed iliofemoral venous segments had the lowest ambulatory venous pressure and the fewest post-thrombotic symptoms long term,90,91,92 indicating that the long-term benefit of treatment is directly related to eliminating thrombus from the deep venous system and maintaining its patency.
Proper catheter positioning within the thrombus is important for a successful lytic outcome. However, once lysis is complete, patency depends upon correction of an underlying iliac stenosis, if one exists. A persistent lesion leads to an unacceptable high rate of re-thrombosis.
Urokinase was the preferred plasminogen activator until 1999, when it was removed from the market by the FDA because of concerns about viral contamination during the manufacturing process. With rt-PA, results are at least equivalent if not better than those obtained with urokinase. The duration of lysis appears shorter without any increase in bleeding complications.
Bjarnason et al93 reported their 5-year experience treating iliofemoral DVT in 87 lower extremities. They reported a technical success rate of 79%. Patients with acute DVT (< 21 days) enjoyed an 85% success compared to only 42% of those who had chronic DVT (> 21 days). Iliofemoral DVT was more successful than femoral popliteal DVT (83% vs 63%), and patients with an underlying malignancy had a significantly worse primary patency (41% 2-year patency rate compared to 75% in patients without malignant disease). They experienced six major complications.
Mewissen et al94 summarized the results of a prospective national venous registry. Two hundred and twenty-one patients with iliofemoral DVT and 79 with femoral popliteal DVT were treated with urokinase infusions. Complete clot lysis was observed in 31% of the infusions, 50-99% lysis in 52% and ≤ 50% lysis in 17%. Thirty-four percent of patients with acute DVT had complete clot lysis, whereas only 19% of patients with chronic DVT had patency restored. Seventy-nine percent of limbs with complete lysis remained patent at one year compared to 58% of limbs with 50-99% lysis and 32% of limbs with ≤ 50% lysis. Patency at one year was significantly better in patients treated for iliofemoral DVT compared to those treated with femoral-popliteal DVT (64% versus 47%, respectively). No patient with femoral-popliteal DVT for more than 10 days prior to treatment achieved complete clot lysis. Following lysis, 99 iliac and 5 femoral vein stenoses underwent angioplasty and stenting. It was apparent that correction of any residual iliac vein lesion improved patency. At one year 74% of the limbs treated with balloon dilation and stenting remained patent as compared with 53% of the limbs without angioplasty and stenting. Stenting was not helpful in the femoral-popliteal segment and four of the five stents placed in that location rethrombosed early (mean of 42 days), with the remaining patient lost to follow-up. Major bleeding complications occurred in 11%. Thirty-nine percent of the bleeding complications occurred at the venous puncture site and 13% were the result of a retroperitoneal bleed. Twenty-eight percent of the patients bled from other sites including musculoskeletal, gastrointestinal or genitourinary systems. There was one fatal intracranial hemorrhage and one patient suffered a subdural hematoma, for a frequency of major neurologic complications of 0.4%. Pulmonary emboli during infusion occurred in six patients (1%), a frequency comparable to patients treated with anticoagulation. One patient suffered a fatal pulmonary embolism; therefore, two patients died as a direct result of catheter-directed thrombolysis for a mortality rate of 0.4% in this series.
The venous registry offered an opportunity to evaluate whether catheter-directed thrombolysis had a beneficial long-term effect on patients compared to standard anticoagulation. An 80-item health related quality-of-life questionnaire was developed that contained items assessing demographics, clinical history, clinical functioning and well-being as well as specific DVT-related items.95 The questionnaire was administered to 98 patients with iliofemoral DVT treated at least 6 months earlier.96 Sixty-eight patients were identified through the national DVT registry and were treated with catheter-directed thrombolysis, and 30 patients were treated with standard anticoagulation for their iliofemoral DVT. Patients randomized to thrombolysis were younger (53 years) than the heparin treatment group (61 years); however, all heparin treated patients were candidates for thrombolytic therapy (no contraindication). Patients who received catheter-directed thrombolysis reported better overall physical functioning (P = .046), less stigma (P = .033), less health distress (P = .022) and fewer post-thrombotic symptoms (P = .006) compared to patients treated with anticoagulation alone. Within the lytic group, phlebographically successful lysis correlated with an improved health related quality of life (P = .038). Patients who failed thrombolysis had a similar quality-of-life outcome to heparin-treated patients.
In summary, catheter-directed thrombolysis can be recommended for patients with symptomatic iliofemoral DVT. rt-PA is currently used as a 4-5 mg bolus followed by an infusion of 2-4 mg/hr. Once lysis is complete, any residual iliac vein lesion must be promptly corrected with balloon angioplasty and stenting if necessary. Successful lysis is associated with a reduction of post-thrombotic morbidity and improves health-related quality of life. It appears that patients with iliofemoral deep venous thrombosis who are not treated with catheter-directed thrombolysis (or iliofemoral venous thrombectomy) have missed an opportunity to preserve deep venous function and avoid serious post-thrombotic consequences.
III. Managing Oral Anticoagulant Therapy: Optimizing Patient Outcomes
Jack E. Ansell, M.D.*
Department of Medicine (E113), Boston University School of Medicine, 88 E Newton Street, Boston MA 02118-2393
The coumarin-type oral anticoagulants have been in use for over 50 years and have remained critically important drugs in the primary and secondary prevention of thromboembolism. In the last 20 years, their use has expanded commensurate with an increased understanding of the important role of thromboembolism in cardiovascular disorders. Considerable efforts have also been applied to improving patient outcomes by identifying the appropriate indications for treatment and the appropriate therapeutic range through large randomized controlled trials, and by improving prothrombin time (PT) results reporting through the use of the International Normalized Ratio (INR). Unfortunately, the benefits of these improvements have not always been realized because of poor patient and dose management leading to a high incidence of serious adverse events. This review emphasizes the important risk factors associated with adverse events and describes the critical factors and models of management that achieve the best outcomes.
Risk Factors for Adverse Events
Clinicians must be aware of how major adverse events are defined when interpreting and comparing the results from clinical trials since precise estimates can only be made if there is consistency between classification schemes in clinical reports.97 Most investigators divide adverse events into minor and major categories with major events including fatal or life threatening bleeds (e.g., intracranial or retroperitoneal) or bleeding with a defined drop in hemoglobin, leading to transfusion of a specified number of units of blood, or leading to hospitalization.
Intensity of treatment
The most important factor influencing the risk of bleeding is the intensity of anticoagulant therapy.98,99,100,101,102,103,104,105,106,107 Four randomized studies (Table 5) have specifically demonstrated that the risk of clinically important bleeding is reduced by targeting a lower therapeutic range.98,99,100,101 Additional studies have shown what amounts to an exponential increase in hemorrhagic events as the INR increases above 5.0.102,104,105,108
Patient characteristics
Several patient characteristics have also been associated with a higher risk of bleeding during anticoagulation.97,102,106,107,108,109,110,111,112,113,114,115,116,117 The patient factor most consistently demonstrated to be predictive of major bleeding is a history of bleeding (especially gastrointestinal bleeding).97,106,112 Other factors include a history of stroke and the presence of a serious comorbid condition such as renal insufficiency, anemia or hypertension.
The relationship between older age and anticoagulant associated bleeding is controversial. Reports suggest that older individuals are not at increased risk for bleeding,97,109,118,119,120,121,122,123,124,125,126,127,128 while others describe such an association.102,106,108,113,115,129,130,131,132 Whether age is simply associated with co-morbid conditions, which themselves are risk factors for bleeding (e.g., colonic polyps, concomitant medications, or poor anticoagulant control due to lack of compliance) or is directly causal is difficult to determine. Studies indicate that older patients who have high quality anticoagulation management, such as that provided by an anticoagulation clinic, have the same risk of bleeding as their younger counterparts.133 Some studies that attempted to separate the effect of age from co-morbid conditions associated with age concluded that age in and of itself is not a major independent risk factor,97,120,134,135 while others have found it to be an independent risk factor102,107 even after controlling for the intensity of the anticoagulant effect. Individuals who are otherwise good candidates for anticoagulation should not have anticoagulation withheld because of their age. However, elderly patients should be monitored more carefully in order to maximize their time in therapeutic range.
Time in therapeutic range
A strong relationship between time in therapeutic range (TTR) and bleeding or thromboembolic rates has been observed across a large number of studies with different patient populations, different target ranges, different scales for measuring intensity of anticoagulation (i.e., PT, PT ratio, and INR), and different models of dose management. 104,105, 108,118,121,136,137,138,139,140 Time in therapeutic range must be interpreted with caution across studies because different methods are used to measure TTR. These include expressing TTR as a rate (% of INRs that are therapeutic or % that are therapeutic at one point in time), or as actual days spent in or out of range (linear interpolation method of Rosendaal et al141 ). The results of all of these methods are influenced by the choice of target INR range and therefore, the TTR can be enhanced considerably if the target range is expanded, even in the absence of any actual improvements in the quality of anticoagulation management. Another deficiency is that the various methods of assessment treat small departures from target range as identical to large departures, even though the former would have much less impact on clinical outcomes than the latter. These differences must be taken into account when comparing results across multiple studies. Table 6 summarizes data from studies where the quality of anticoagulation as reflected by TTR was assessed.
Frequency of testing
The optimal frequency of monitoring the INR is dependent on many factors, including patient compliance, transient fluctuations in comorbid conditions, the addition or discontinuation of other medications, changes in diet, the quality of dose adjustment decisions, and whether treatment is early or late in the course of therapy. Recent clinical trials suggest that time in therapeutic range, and presumably fewer adverse events, can be maximized by more frequent testing.142,143 This is particularly true in studies utilizing patient self-testing with point-of-care (POC) devices where access to testing is virtually unlimited. Horstkotte et al142 specifically addressed this issue in 200 patients with mechanical cardiac valves where he found that the percent of INRs within target range varied from 48% when monitoring occurred at an average interval of 24 days, to 89% when monitoring was at an average of every 4 days. It is difficult to find studies where investigators report both TTR as well as achieved frequency of testing. The studies highlighted in Table 6 indicate the few reports where this information was provided and shows a direct correlation between increased frequency of testing and TTR.
Managing the Risks of Oral Anticoagulant Therapy
Although several developments have improved the safety and efficacy of oral anticoagulation, less has occurred to substantially improve the management of oral anticoagulation. There is now growing evidence that coordinated programs focused on anticoagulant management achieve a high rate of maintaining patients in therapeutic range leading to improved outcomes.144
Anticoagulation Management Services
A systematic approach to the management of therapy by specialized programs (anticoagulation clinics) significantly improves clinical outcomes by improving time in therapeutic range, lessening the frequency of hemorrhage or thrombosis, and decreasing the use of medical resources leading to more cost-effective therapy. These programs are characterized by a knowledgeable provider who manages therapy, an organized system of follow up, reliable prothrombin time monitoring, and good patient communication and education.
Most patients today are managed by their personal physician along with all other patients in their physician's practice without an organized program of management, education or follow-up (designated “usual care”).106,112,116 Table 7 summarizes the few studies assessing clinical outcomes in this “usual care” model of management. These studies indicate a combined rate of major hemorrhage and of recurrent or de novo thromboembolism of 15% per patient year of therapy. These adverse events are generally a consequence of poor therapeutic control with hemorrhage or thrombosis occurring as a consequence of excessive or subtherapeutic anticoagulation. These outcomes can be contrasted to the rates identified in a large number of retrospective and some prospective studies of care delivered by an anticoagulation service (Table 7).97,104,108,115,118,119,120,121,122,145,146,147 These studies indicate a more than 40% reduction in both major hemorrhage and thrombosis compared to usual care. Lastly, Table 7 summarizes studies examining both models of management where coordinated care is measured against a control group of usual care within each study.114,148,149,150 These non-randomized, retrospective analyses provide further evidence for the benefit of coordinated care.
A few studies also suggest a significant cost benefit to coordinated vs. usual care by a reduction in adverse events and reduced utilization of hospital services. Gray et al151 estimated a savings of $860 per patient year of therapy due to reduced hospital days per patient year. Chiquette et al150 reported cost savings through a coordinated approach compared to usual care of approximately $1,320 per patient year of therapy.
Patient Self-Testing and Patient Self-Management
As a result of technological advances in prothrombin time measurement, there is potential for further simplifying and improving anticoagulation management by POC testing. POC testing allows for the determination of a prothrombin time from a fingerstick sample of whole blood and consequently opens the possibility for patient self-testing. Portability of instrumentation means that prothrombin time measurements are no longer confined to the physician's office, a private laboratory or a nearby hospital, but can be moved into the patient's home or even taken with the patient when traveling. Standardization of reagents and instruments, as well as reliance on the INR, further reduces the inaccuracies of multiple reagents and laboratories.
Since the late 1980s, a number of instruments have been introduced or are in development (Table 8). These instruments are based on clot detection methodology using thromboplastin to initiate clot formation, while the endpoint of clot detection varies from instrument to instrument. The validity of this methodology was initially established in 1987 by Lucas et al152 who used the Biotrack instrument (Coumatrak; Biotrack Inc; Freemont, CA) and showed a correlation coefficient of 0.96 between reference plasma PTs and capillary whole blood PTs. Within-day precision using two different levels of controls revealed coefficient of variations of 4.9% and 2.9%. Similar results have been obtained by others,153,154 but some investigators have identified limits of comparability due to the high International Sensitivity Index (i.e. low sensitivity) of one company's thromboplastin, as well as the inability to determine a true geometric mean normal prothrombin time for the instrument.155,156 Studies by McCurdy and White157 indicated that the INR correlation was adequate within an INR range of 2.0-3.0 (the therapeutic range for most indications), but began to lose its comparability as the INR increased above the 4.5 range. A recent comparative study of nine different POC devices showed that all devices correlated well with different laboratory methods and thromboplastins, but most instruments showed significant differences from the laboratory results.158 This study illustrated the limitations of using local reference material and the need to know each instrument's bias compared to a local laboratory if that is the reference being used.
Although the appropriate studies have not been done, it is possible that this new system is significantly more reliable and consistent than the variable and haphazard PT monitoring often employed in the usual care of most patients on oral anticoagulants.159
A number of studies have demonstrated the ability of patients to perform self-testing and obtain an accurate result.160,161,162 Patients can then call their physician and obtain instructions for warfarin dose adjustment. Table 9 summarizes those studies where clinical outcomes, either time in therapeutic range or adverse events, have been reported. Patient self-testing can also incorporate patient self-management of warfarin dosing, and a number of studies have shown this model of care to be safe and effective as well.143,163,164,165,166
Based on the foregoing, POC PT monitors offer the potential to improve the safety profile of anticoagulant therapy; to improve patient satisfaction and possibly patient compliance; and by reducing the labor intensity of physician management, to encourage the more widespread use of warfarin. The cost-effectiveness of such therapy needs to be studied.
Data Management and Computerized Dosing
An obstacle to the safety and effectiveness of warfarin therapy is the poor quality of dose management as currently practiced.144 As indicated in Table 6, there is a wide range of success in achieving TTR. A usual care model appears to yield the worst results with a TTR between 33-64%. Even in randomized controlled trials where patient care is often highly structured, TTR varies between 48-83%. Achieved TTR appears to be best in either an anticoagulation management service model or with patient self-management (approximately 60-90%). Computer assistance by the use of dedicated programs may, however, improve dose management and time in range.
The first randomized study of computerized dosing in 1993167 showed that three contemporary computer programs all performed as well as an experienced medical staff of an anticoagulation management service in achieving a target INR of 2.0 to 3.0, but the computer achieved significantly better control when more intensive therapy was required (INR 3.0 to 4.5). In another randomized study168 of 101 long-term anticoagulated patients with prosthetic cardiac valves, computerized warfarin adjustments proved comparable to manual regulation in the percentage of INR values maintained within the therapeutic range, but required 50% fewer dose adjustments. The first multicenter randomized trial of one computerized dosage program in 1998169 showed a 22% overall improvement of control with the program (Dawn AC®) compared to performance by the medical staff. The computer program gave significantly better INR control than experienced medical staff for all 285 patients for all target INR ranges. The study also showed that the natural increased caution of medical staff in dosing patients at a higher INR range is not shared by the computer. It cannot be assumed, however, that all computer programs will be equally successful, and new programs will require independent validation by randomized controlled studies to determine the extent of their ability to accurately predict dosage control.