Abstract
This review addresses several areas of concern in the care of patients with sickle cell disease. In Sections I and II, the fundamental pathogenetic mechanisms of sickle cell disease and their clinical consequences are discussed. Dr. Narla presents the evidence for abnormal cell adhesiveness by SS cells and Dr. Rosse examines the role of the increased whole blood viscosity. In Section III, Dr. Petz reviews common and uncommon alloimmune consequences of transfusion in sickle cell disease and discusses the diagnosis and management of sickle cell patients with hyperhemolysis after transfusion. In Section IV, Dr. Steinberg gives an update on the use of hydroxyurea in the treatment of sickle cell disease, including the SC and S-β thalassemia variants.
I. Sickle Cell Adhesion
Mohandas Narla, D.Sc.*
Life Sciences Division, Lawrence Berkeley Laboratory, 1 Cyclotron Road, Bldg. 74-157, Berkeley CA 94720
An important pathophysiologic feature of sickle cell disease is episodic occurrence of vasoocclusive events that precipitate acute painful episodes. Vasoocclusion of small and sometimes large vessels is the hallmark of sickle cell disease, accounting for much of its morbidity and mortality. Decades of research on sickle hemoglobin polymerization have culminated in elegant elucidations of the contributions of polymerization-dependent processes to various pathophysiologic manifestations of the sickle cell disease. However, it is reasonable to surmise that hemoglobin polymerization in and of itself is not sufficient to account for the episodic nature of vascular occlusion.
Based upon studies from a number of laboratories, there is emerging consensus that a key contributor to vasoocclusion may be the increased tendency of sickle red cells to adhere to vascular endothelium.1,2 Vasoocclusion can occur when transit time of red cells through the capillaries is longer than the delay time for deoxygenation-induced hemoglobin polymerization of sickle hemoglobin. As adherence of sickle red cells to vascular endothelium will impede blood flow and thereby increase capillary transit time, it has been suggested that increased cell adherence can initiate and propagate vasoocclusion.
Factors such as inflammatory mediators that activate endothelial cells and thereby enhance endothelial adhesivity of sickle red cells thus have the potential to trigger vasoocclusive episodes. A partial list of agonists that may alter endothelium and play a role in sickle cell disease includes TNF-α, interferon-γ, IL-1β, vascular endothelial growth factor (VEGF), thrombin, and histamine, and the effects of hypoxia and reperfusion.
Seminal studies by Hebbel two decades ago demonstrated that sickle red cells exhibit increased adherence to endothelial cells in vitro and that the extent of in vitro sickle cell adhesivity correlated with vasoocclusive severity.3 Mohandas and Evans showed that both red cell membrane changes and plasma factors account for increased sickle cell adherence to endothelial cells in vitro.4 Subsequently, a number of studies have defined adhesion pathways involved in sickle cell adherence to cultured endothelial cells under static and flow conditions.5,6,7,8,9,10 Adhesive ligands identified on sickle red cells include CD36, α4β1 integrin, sulfated glycolipid and the Lutheran blood group antigen. On the endothelial side, cytokine-induced VCAM-1, a ligand for α4β1, and αvβ3 integrin that binds von Willebrand factor (vWf) and thrombospondin (TSP) have been shown to mediate sickle cell adherence. Other potential adhesive receptors on endothelial cells include GPIb and CD36. The adhesive proteins in plasma, TSP released by platelets, and vWF released by endothelial cells mediate adhesion by serving as bridging molecules between adhesive receptors on red cells and endothelial cells. Sickle red cell interaction with the vessel wall may also involve interaction with subendothelial matrix components such as laminin, TSP, vWf or fibronectin exposed by vascular injury. The Lutheran blood group antigen has been shown to be a major laminin receptor on red cells, while a sulfated glycolipid has been shown to bind to laminin and to TSP. Based on data from these extensive series of in vitro studies it is reasonable to conclude that sickle red cells indeed exhibit an increased adhesive phenotype and that a large number of cell adhesion receptors, plasma proteins and subendothelial matrix components are involved in mediating adhesive interactions.
The critical question that has not yet been adequately addressed is the extent to which the in vitro documented adhesive phenotype of sickle red cells contributes to vasoocclusion in vivo. While there are no easy experimental strategies to address these problems, some progress is being made through physiological studies using animal models. Intravital microscopy has been employed to study sickle red cell interaction with vessel wall using rat mesocecum ex vivo perfused with human sickle cells and in transgenic mice that express high levels of human sickle hemoglobin.11,12,13 The ex vivo rat studies showed that deformable low-density sickle red cells are more likely to adhere than undeformable dense sickle cells and that adhesion was limited to postcapillary venules. A recent study using the same ex vivo rat model has convincingly demonstrated that treatment with the αvβ3-blocking antibody largely abolished platelet-activating factor-stimulated sickle red cell adhesion to vessel wall.14 These types of in vivo studies are beginning to provide support to the thesis that increased sickle cell adherence could have significant effects on flow dynamics in the microvasculature and that anti-adhesive therapy may have clinical benefits.
There are, however, a number of significant issues regarding the in vivo role of sickle cell adherence that cannot be addressed using ex vivo animal models. A major hurdle for progress has been the lack of a suitable animal model for sickle cell disease. The recent development of transgenic/knockout sickle mice that express exclusively human sickle hemoglobin and exhibit many clinical features of human disease15 is likely to prove to be a valuable tool to begin to critically evaluate the role of cell adherence in vasoocclusion and the potential clinical benefit of anti-adhesive therapeutic strategies. While much work still remains to be done, with the recent exciting breakthroughs in our understanding of cell adhesion it is likely that anti-adhesion therapies may become viable treatment options for management of vasoocclusive crisis during the coming decade.
II. Blood Viscosity in Sickle Cell Disease
Wendell F. Rosse, M.D.*
Duke University Medical Center, PO Box 3934, Durham NC 27710
Clearly, the symptoms and major effects of sickle cell disease result from the non-delivery of oxygen by abnormal blood. Much emphasis has been placed on the role of vasoocclusion (see Section I). On the other hand, oxygen delivery may be impaired because of increased blood viscosity without primary adhesion and occlusion as envisioned in the model that has been outlined. This section will focus on the role of whole blood viscosity in the pathophysiology of sickle cell disease.
The viscosity of a fluid is defined as the resistance to flow of that fluid and is determined by a number of factors, including temperature and the intrinsic properties of the fluid. In simple, so-called Newtonian fluids, viscosity is not dependent on the shear rate applied to the fluid. Blood differs in that the viscosity decreases with increasing shear rate. Flow of a viscous fluid through an orifice or pipe is dependent upon the viscosity of the fluid, the force applied to the fluid, and the diameter of the orifice; all of these are important in analyzing the role of whole blood viscosity on the delivery of oxygen.
Blood consists of a suspension of particulate cells in a fluid phase. Whereas the fluid plasma can be analyzed in the Newtonian equations, the addition of particles which themselves have differences in internal and membrane viscosity renders a priori analysis difficult at best. Nevertheless, the viscosity of the elements of blood and the viscosity of the whole blood suspension can be analyzed and related to the clinical effects on blood flow in normal and abnormal clinical states, particularly sickle cell disease.
The Viscosity of Individual Red Cells
The red cells in sickle cell disease have been studied in a number of ways in order to define the intrinsic characteristics that are altered in sickle cell disease.16,17,18 These studies all indicate that the membrane viscosity and deformability in sickle cell disease is markedly altered even when the cell is fully oxygenated. Greater forces are required to cause deformation of the membrane and the internal viscosity of the red cells is increased even at resting steady state and is markedly increased when the hemoglobin is deoxygenated.19,20,21,22 This is easy to understand since the molecules of hemoglobin are no longer randomly arranged when this occurs but rather form structured, elongated arrays. The increase in viscosity is greater when deoxygenation is sudden and rapid than when it is gradual.23 These studies can provide a basis for estimating the effects of hemoglobin concentration and composition in various clinical states.
The increased erythrocytic intracellular and membrane viscosity translates directly into increased whole blood viscosity. In studies comparing sickle cell and normal blood at the same hematocrit, the measured viscosity of the oxygenated sickle blood was 1.5-fold that of normal at equal shear rates16 but was increased to 10-fold that of normal blood on deoxygenation. The viscosity decreased linearly as the proportion of HbSS cells was reduced by mixing, but the increase in viscosity remained significant even at levels of 25% HbS.24
The Role of Hematocrit
While the internal viscosity of the red cells is of great importance in determining the flow characteristics of the blood, a major determinant of whole blood viscosity is the hematocrit of the blood. For normal blood, the viscosity rises linearly as the hematocrit is increased. At very high hematocrits, the viscosity may rise at a greater rate than the rate of rise of the hematocrit, although this is questioned. More importantly, the delivery of oxygen began to decrease above hematocrits of 45-50% and is dramatically decreased at a hematocrit of 60%. This decrease reflects the diminished circulation in small vessels as the whole blood viscosity rises.
The effect of hematocrit is even more striking when blood containing HbS is examined. At full oxygen saturation, the curve relating viscosity to hematocrit was much steeper than for normal; deoxygenation made it steeper still. The curve relating oxygen delivery to hematocrit was strikingly shifted such that, even with full oxygenation, the diminution in oxygen delivery begins at hematocrits of 30-35% and is striking at hematocrits of 45%. When the blood was even minimally deoxygenated, the curve was shifted even further. Diminished oxygen delivery results in tissue hypoxia and further desaturation of the hemoglobin in a vicious cycle.
The Effects of Increased Viscosity
The effects on the circulation due to increased viscosity are seen in larger vessels than the effects of adherence and occlusion. The rate of flow through a tube is governed by the diameter of the tube, all other things being equal. With increased viscosity, the flow is diminished and the diminution increases as the diameter decreases. In the circulatory system, the critical diameter is probably at the level of the arterioles since the effects of increased viscosity appear to be manifest in the hypoxia and consequent changes in function of tissues supplied by vessels of this caliber. If the consequent hypoxia is sufficiently prolonged in a sufficiently large volume of tissue, necrosis results.
A second and important effect of increased whole blood viscosity is the increased tendency to thrombosis, probably due to the slowed rate of circulation. This thrombosis can be seen on either the arterial or venous side of the circulation and has been known for many years in the pathophysiology of polycythemia and macroglobulinemia. These effects are exaggerated in the case of sickle cell disease.
Clinical Complications Made Worse by Increased Viscosity
It is difficult at best to observe the changes to blood flow in vivo in patients with sickle cell disease. However, the effects of increased viscosity can be assessed by noting the complications in which high hematocrit is a risk factor. Since the main effect of an increased hematocrit in sickle cell disease is a striking increase in blood viscosity, this measure serves as a surrogate for the effect of increased viscosity in the pathogenesis of the disease.
Acute complications
Acute Chest Syndrome: The results of the Cooperative Study of Sickle Cell Disease (CSSCD) demonstrated that, in adults, an increased hematocrit was a major risk factor in the genesis of the acute chest syndrome.25 While the term is somewhat over-inclusive and may encompass pneumonia, fat embolism, thromboembolism, etc., a major cause or component in most cases is altered circulation of the blood through the pulmonary circuit. At least part of the problem may be an inability to de-sickle incoming venous blood; this would lead to an increase in viscosity, further decreasing the ability of the blood to circulate. This appears to occur in small patches, which then coalesce into regions of altered ventilation-perfusion and hypoxia. The areas of the lung beyond this circulatory obstacle are prone to infection, resulting in pneumonia. If the area affected is large enough and the hypoxia sufficient, destruction of the lung occurs. In this scenario, it is easy to see how an increased hematocrit would be a liability.
Acute Multi-Organ Damage Syndrome: A syndrome in which one or more organs are damaged at the same time has been described particularly in patients with high hematocrit.26 The onset is due to arterial hypoxia, such as may be generated by the acute chest syndrome, but often there is no apparent predisposing event other than sudden syncope. The symptoms are due to insufficient perfusion of one or more organs. The effect on the brain usually results in non-focal coma, and imaging studies may be of little help in elucidating the difficulty; in this respect, the effects resemble those of thrombotic thrombocytopenic purpura. Recovery of cerebral function may take several days but is usually complete or nearly so. The kidneys may be affected, manifested by hematuria or acute renal failure. Acute necrosis of the liver or hepatic sequestration syndrome may occur. The bone marrow may be generally affected with necrosis of the marrow and generation of fat emboli. Pancreatitis may occur. These evidences of tissue damage can occur in any combination and may persist for 1-2 weeks.
This syndrome is most common in sickle cell disease and S-β thalassemia but does occur in patients with classical SS disease and a high hematocrit. The genesis is presumably diminished arterial input due to the increased viscosity of the hypoxic blood. The treatment is the emergent replacement of the blood with blood of lower viscosity
“Watershed” Stroke: Cerebrovascular events can occur in several forms in sickle cell disease. One type appears to be a special case of the events noted in the multi-organ damage syndrome, in which the circulation to an area of the central nervous system is impaired sufficiently to cause necrosis. The obstruction to flow occurs in the terminal branches of the circulatory system; hence, the resultant defects are not as global as those cause by occlusion of major vessels. The area affected can usually be demonstrated by careful imaging, especially with magnetic resonance imaging (MRI).
Chronic complications
Pain: All pain and pain episodes in sickle cell disease are not the same. The classic “vasoocclusive crisis,” due presumably to the adhesion of erythrocytes to the endothelium with consequent occlusion of the microcirculation beyond, is usually sudden in onset, lasts 5-10 days followed by a gradual offset, and is then over until the next episode occurs. It is marked by the generation of products of coagulation (D-dimer, prothrombin fragments, cross-linked fibrin monomer, etc.), perhaps consequences of events stabilizing the occluding barrier. In patients with homozygous sickle cell disease, three factors correlate with the frequency and onset of such events: infections (which may alter the characteristics of the plasma), leukocytosis (which may slow flow in the microcirculation, particularly during infection), and increased hematocrit; the latter effect is, however, minor.
Some patients with syndromes of sickle cell disease characterized by higher hematocrits (sickle cell disease, S-β thalassemia, etc.) have fewer typical vasoocclusive crises but may have pain that is more transient, not as severe, and not accompanied by evidence of coagulation. In some, there may be subcutaneous edema and great tenderness of the subcutaneous tissues, due perhaps to diminished oxygen delivery to these areas. These patients are often quite incapacitated by the frequency of the pain and are likely to become frequent users of pain medications. Because they have supposedly milder forms of sickle cell disease, their symptoms are sometimes ignored. This type of pain is presumably due to the effects of increased viscosity, which leads to reduced oxygen delivery and subsequent hypoxia. In some patients, reduction in the hematocrit may lead to improvement in the discomfort.
Aseptic Necrosis of Bone: The aseptic necrosis of the proximal epiphyseal segments of the humeri and especially the femora is a relatively common event in sickle cell disease27 and is more common in patients with HbSS and α thalassemia or in patients with S-β thalassemia. The pathogenesis is not entirely clear but is probably related to the fact that these structures are essentially enclosed spaces with a single incoming artery and outgoing vein. One suggested scenario posits that the increased viscosity of blood retards the venous outflow, resulting in an increase in the tissue pressure within the enclosed space. This pressure then begins to reduce arterial input with resultant hypoxia of the marrow and bone.
Ocular Complications: Decreased circulation to the retina results in a series of defects related to hypoxia, including so-called “salmon spots,” retinal degeneration, and neovascularization said to resemble a sea fan. These are presumably due to difficulties in circulation through arterioles of the size that can be observed on the retina. While these findings are common enough in SS disease, their incidence is related to an elevation of the hematocrit and whole blood viscosity28 and they are common in sickle cell and S-β thalassemia. If the retinal vessels are a reflection of what happens in other small-sized vessels, such changes may be more general than is usually appreciated.
Hypertransfusion syndrome
When the hematocrit is elevated too rapidly or too high in the course of transfusion, several complications may result, including heart failure from cardiac overload. In addition, some of the syndromes listed above may come from the increase in viscosity because of the increase in hematocrit; the most common of these complications is a stroke. Even though the blood being transfused will contribute less to the viscosity than the circulating blood, the increase in hematocrit may be sufficient to increase the viscosity to dangerous levels.
Implications for Therapy
If hyperviscosity is playing an important role in the pathogenesis of an acute complication of sickle cell disease, then therapy should be directed at the rapid reduction of it. This is best done by exchange transfusion, using a mechanical device to remove and replace the patient's blood. The objective should be a reduction in hematocrit to less than 30% and a proportion of transfused blood greater than 80%. These values can be obtained by using calculations based on the blood volume (estimated from the patient's body weight), initial hematocrit, initial proportion of HbS, final hematocrit, and final desired proportion of HbS. If the initial hematocrit is above 35%, it may be useful to remove a unit or two of the patient's blood before the calculation is done.
In treating the acute chest syndrome, exchange transfusion should be undertaken if there are signs of increasing infiltrate on chest X-ray, if the arterial pO2 cannot be maintained above 70 torr, or if the patient is experiencing oxygen hunger manifest by dyspnea or tachypnea. When this regimen has been followed at Duke, we have had only two deaths in 32 patients with serious acute chest syndrome.
In treating the acute multi-organ damage syndrome, exchange transfusion should be undertaken as soon as possible to prevent damage from being permanent. Supportive care should be continued as long as possible since many patients who appear moribund recover completely or nearly so with sufficient time.
The role of exchange transfusion is not so clear in the “watershed stroke” syndrome, but there is reason to believe that its prompt institution would be beneficial.
The chronic suppression of hyperviscosity is much more difficult. None of the many drugs that have been tried in an effort to reduce hyperviscosity pharmacologically are useful. Reduction of the hematocrit by phlebotomy can be useful; we have tried this on a series of 11 patients with high hematocrit syndromes with reported benefit. This reduction is difficult to maintain and venous access is frequently a problem. At present, there is no pharmacological agent that selectively reduces erythrocyte production; one is needed.
Considerations of the effect of high hematocrit in causing effects from hyperviscosity also come into play when testing drugs directed at the amelioration of sickle cell disease. Drugs that ameliorate the disease will usually elevate the hematocrit. Care must be take to be sure that this effect does not outweigh the beneficial effect of the drug in countering the sickling process.
III. Hemolytic Transfusion Reactions in Patients with Sickle Cell Anemia
Lawrence D. Petz, M.D.*
Pathology and Lab Medicine, UCLA Medical Center, 10833 LeConte Ave., A-6238D CHS, Box 951713, Los Angeles CA 90095-1713
Hemolytic transfusion reactions (HTRs) are of particular concern in patients with sickle cell disease. In addition to the usual laboratory manifestations of hemolysis, patients with sickle cell disease may develop serious and even life-threatening problems. These HTRs may present with a number of distinctive features that form part of a constellation of findings we have termed the Sickle Cell HTR syndrome.29
Components of the Sickle Cell Hemolytic Transfusion Reaction Syndrome
Manifestations of an acute or delayed hemolytic transfusion reaction.
Symptoms suggestive of a sickle cell pain crisis, which develop or are intensified during the hemolytic reaction.
Marked reticulocytopenia (a significant decrease in absolute reticulocyte level compared to the patient's usual value).
Development of a more severe anemia following transfusion than was previously present. A rapid drop in hemoglobin and hematocrit can occur when hemolysis of donor RBC is accompanied by suppressed erythropoiesis since sickle cell RBCs have an intrinsically short survival. In some patients, it is possible that hyperhemolysis of autologous RBC (bystander immune hemolysis) may play a role in causing the decrease in hemoglobin and hematocrit, although more definitive documentation of this phenomenon is necessary.
Subsequent transfusions may further exacerbate the anemia, which may become life threatening or even fatal.
Patients often have multiple red cell alloantibodies and may also have autoantibodies making it difficult or impossible to find compatible units of RBC. However, in other patients, no alloantibodies are demonstrable, or patients may have alloantibodies for which antigen-negative RBC are readily obtainable.
Serologic studies may not provide an explanation for the hemolytic transfusion reaction. Even RBC phenotypically matched with multiple patient antigens may be hemolyzed.
Recovery manifested by reticulocytosis and gradual improvement in hemoglobin may occur only after the withholding of further transfusion. The administration of corticosteroids appears to be an important therapeutic measure in some patients.
After a recovery period, similar symptoms may recur following subsequent transfusions although other patients tolerate further transfusions without incident.
Comments Regarding the Components of the Sickle Cell Hemolytic Transfusion Reaction Syndrome
The development or intensification of symptoms suggestive of a sickle cell pain crisis.
Patients who experience brisk hemolysis often develop generalized malaise, back pain, flank pain, chest pain and fever and, in a patient with sickle cell disease, these findings may be interpreted by the patient and/or the attending physician as being caused by a sickle cell pain crisis. It is not always clear whether the pain symptoms are simply part of the HTR and misinterpreted as a sickle cell pain crisis or whether the hemolysis initiates a true pain crisis. The critical point to keep in mind is that the diagnosis of a HTR may go unrecognized because of the tendency to attribute all signs and symptoms in an acutely ill patient with sickle cell disease to a diagnosis of pain crisis.30,31,32 The delay in making a diagnosis of a HTR contributes significantly to morbidity and to the probability of mortality.
Reticulocytopenia
Another frequent finding in the sickle cell HTR syndrome is reticulocytopenia, which we have defined as a significant decrease in the absolute reticulocyte level compared to the patient's usual value. Reticulocytosis is a critical mechanism by which patients with sickle cell disease partially compensate for their shortened red cell survival. If erythropoiesis is suppressed in patients with a very short RBC survival, a rapid increase in the severity of the anemia will occur. Accordingly, if a patient with sickle cell disease has a severe HTR in which transfused RBC are hemolyzed rapidly and, in addition, the patient's reticulocyte level is significantly depressed, severe and life-threatening anemia may develop.
The development of more severe anemia than was present prior to transfusion
One of the most important findings in the sickle cell HTR syndrome is the development of more severe anemia than was present prior to transfusion, as has been indicated in a number of reports.29,31.33.34 This presents a therapeutic dilemma since a patient with severe anemia may seem to require transfusion, yet the anemia may become progressively more severe after each transfusion.29,31,35 The following case report illustrates this point.29
A 28-year-old woman with sickle cell anemia was hospitalized with chest, knee, and back pain, diarrhea and an ankle ulcer. Her hematocrit (Hct) on Day 2 was 13.2%. She was transfused with 3 units of RBCs over the next 5 days, after which her Hct rose to 24.7%. However, signs of a delayed hemolytic transfusion reaction (DHTR) developed, and on Day 9, she received three additional units of RBCs. On Day 12, after having received a total of 6 units of RBC, her hematocrit had fallen to 11%. During the next three days, she received an additional 9 units of RBC but, after a temporary rise, her Hct dropped further to a level of 9.3% on Day 17. Thus, after the transfusion of a total of 15 units of RBC, her hematocrit had gone from 13 to 9.3%! It should be noted that during the first hemolytic episode her reticulocytes dropped to a nadir of 4.5% and, during the second episode, to 2.8%.
Prednisone (60 mg/day) was begun on Day 15 and two more units of RBCs were transfused on Day 17. Subsequently, signs of hemolysis decreased and the Hct progressively increased. Twenty-seven months later she manifested similar findings after transfusion.
Serologic Findings
The sickle cell HTR syndrome most often occurs in patients who have multiple RBC alloantibodies, at times in association with autoantibodies. In some patients, a newly detected RBC alloantibody develops following transfusion as is typical in a DHTR, whereas in other patients the serologic findings do not provide an explanation for the hemolysis. This may be because no new antibodies become apparent during the DHTR or, in some instances, because there are no alloantibodies or autoantibodies demonstrable at any time.
A classic case was reported by Chaplin and Cassell,36 who described, in remarkable detail, a patient with sickle cell disease in whom rapid destruction of transfused red cells occurred repeatedly despite entirely compatible crossmatch results by a wide variety of serologic methods. Overt hemolysis occurred even after the transfusion of crossmatch-compatible RBCs from two siblings whose blood types were identical to the patient's with respect to numerous RBC antigens. These authors were the first to point out the regular onset of typical sickle pain “crisis” coincident with the rapid destruction of large volumes of donor erythrocytes.
Diamond et al30 and Cullis et al32 also described patients who had clinical and laboratory findings of a delayed HTR, although the direct antiglobulin test (DAT) and antibody screen remained negative.
Although DHTRs in sickle cell patients have been reported in which serologic findings do not explain the hemolysis, one must not think of this phenomenon as unique to sickle cell patients. Cases of DHTR have been described in patients without sickle cell disease in which the DAT remained persistently negative and no new alloantibodies were detected in the serum37,38
Management
The most difficult aspect of management is the question of transfusing a patient who has had a HTR following which the anemia has become more severe than it was prior to transfusion. Subsequent transfusions may further exacerbate the anemia and it may become life-threatening or even fatal.31,35 Transfusion may be absolutely necessary in some patients but, if practical, withholding transfusion and treating with corticosteroids, possibly in association with IVIG and large doses of erythropoietin, is often preferable. Very close observation of severely anemic patients is required to make such judgments appropriately.
Possible Mechanisms of Development of More Severe Anemia Following Transfusion
Progressively more severe anemia following transfusion could be due to hemolysis of donor RBCs coupled with suppression of erythropoiesis. Alternatively, extreme posttransfusion anemia could be due to an immune reaction that causes in increased rate of hemolysis (hyperhemolysis) of the recipient's own RBCs. This would be an example of a type of hemolytic reaction that has been termed “bystander immune cytolysis.”29,39
Suppression of erythropoiesis
When patients with sickle cell anemia develop suppression of erythropoiesis, as may be caused by infection, a marked drop in Hct is to be expected because of the short survival of the patients' RBCs. Such an abrupt drop in hemoglobin and hematocrit may be mistaken for hyperhemolysis. Indeed, transfusion itself suppresses erythropoiesis, as has been well documented in patients with sickle cell disease by Donegan et al.40 Therefore, when there is an unexpectedly low Hct after transfusion, it is important to calculate the magnitude of the drop that can be explained on the basis of suppression of erythropoiesis, so as to not inappropriately interpret the drop in Hct as indicating hyperhemolysis.
The magnitude of a drop in Hct that can be explained on the basis of depressed erythropoiesis may be determined from the expected loss of RBC volume through senescence and the residual RBC production as indicated by hematologic data including reticulocyte counts incorporating known correction factors for reticulocyte maturation. We performed such calculations in a series of sickle cell patients,29 and the findings in one such patient are as follows:
A 22-year-old man with sickle cell anemia was admitted with severe back pain, productive cough, dyspnea, fever and purulent sputum. The Hct on admission was 25.9% and over the next seven days it dropped to 13.9%. The patient had no evidence of bleeding and he was not transfused during this period. Of particular note is that the uncorrected reticulocyte count dropped from 18.1% to 3.2% during this time. Using a figure of 69 ml/kg for blood volume,41 the patients weight of 66 kg, and the patient's hematocrit, we estimated the patient's RBC volume on admission as 1,179 mL. Using hematologic data on admission as an estimate of steady state conditions, we calculated that RBC production and destruction would be 107 mL per day. Over a 7-day period 749 mL of RBC would have reached the end of their life span. If RBC production had ceased, there would have been a loss of 749 mL, leaving a RBC volume of 430 mL. In this case, the patient's hematocrit would have been only 9.3%. Actually, the patient's hematocrit on day 7 was 13.9%, indicating that RBC production had not ceased entirely. Most significantly, the calculations indicate that suppressed erythropoiesis in this patient with sickle cell disease could readily account for the alarming fall in Hct from 25.9 to 13.9% over a 7 days.
Bystander immune hemolysis.
Bystander immune cytolysis (BIC) may be the mechanism by which the patient's own RBC are hemolyzed during a HTR. BIC may be defined as immune cytolysis caused by an alloantigen-induced immune response leading to lysis of cells that do not contain the antigen as an intrinsic part of their membrane. BIC has been reported to occur in a number of clinical settings,39 including the passenger lymphocyte syndrome following minor ABO incompatible marrow transplants,42,43 severe hemolytic transfusion reactions,30,44 posttransfusion purpura,45 and drug-induced immune hemolysis.39 This could lead to an increased rate of hemolysis of the patient's RBCs (“hyperhemolysis”), which has been suggested as the mechanism leading to severe anemia following a HTR in sickle cell anemia patients by a number of investigators.32,33,34,35 ,46,47
A number of possible mechanisms have been suggested as explanations for the pathogenesis of BIC.39 One proposed mechanism is reactive hemolysis that is caused by activated complement components that are formed because of an antigen-antibody reaction. These activated complement components may then attach to RBCs not involved in the original immune reaction, resulting in their lysis.48,49,50 Both Salama and Mueller-Eckhardt51 and Ness et al52 have reported that autologous RBCs are sensitized by complement with or without detectable antibody in at least some instances of delayed HTRs, although in neither study was an effort made to determine if autologous RBCs had a shortened survival. Reactive hemolysis seemed a particularly attractive hypothesis regarding patients with sickle cell anemia because their red cells have an increased susceptibility to lysis by complement.53 However, a syndrome similar to the sickle cell HTR syndrome occurs in patients with thalassemia54 and may occur in patients with other causes of hemolytic anemia.55 Another possible mechanism for destruction of autologous RBCs during a HTR is the development or augmentation of RBC autoantibodies as a result of immune modulation resulting from transfusion. Indeed, a number of authors have attributed severe anemia after transfusion of patients with sickle cell anemia to autoantibodies.29,39,46,56,57
In patients with the sickle cell HTR syndrome, it is difficult to develop definitive data on the mechanism of the severe anemia that may develop. Hyperhemolysis of the patient's RBC is an attractive hypothesis and may well provide the explanation or at least be a contributing factor, but data that are more definitive are required to document this intriguing possibility.39
IV. Current Use of Hydroxyurea in Sickle Cell Disease
Martin H. Steinberg, M.D.*
Boston University, 88 E Newton, Room 211E, Boston MA 02118
More than 50 years ago Janet Watson recognized that the red cells of infants with sickle cell trait failed to sickle in vitro as did cells of their mother's with sickle cell trait and that infants with sickle cell anemia had few symptoms. She hypothesized that these observations were due to elevated fetal hemoglobin (HbF) levels in infant blood. HbF interferes with HbS polymerization and it was appreciated that enough HbF—if evenly distributed among sickle erythrocytes—might “cure” the sickle cell disease. A search was launched for pharmacological agents that could reverse the switch from γ- to β-globin chain synthesis—γ-globin chains characterize HbF and sickle β-globin chains are present in HbS—or select adult erythroid precursors that maintained the ability to produce HbF. The first “hemoglobin switching” agent, a nucleoside analog 5-azacytidine, was postulated to increase HbF by inducing gene expression. Other drugs—hydroxyurea is the prototype—promote HbF production indirectly, perturbing the maturation of erythroid precursors.
Mechanisms of Action of HbF-Activating Agents
Methylation and gene expression
Among the DNA alterations postulated to have an important influence upon gene expression is cytosine methylation and demethylation at CpG dinucleotides.58 Inducing hypomethylation activates transcription of many tissue-specific genes.59 Cytosine methylation may repress transcription.60 In fetal tissues, human γ-globin genes are hypomethylated.61 However, hypomethylation does not accompany all expressed genes; whether gene hypomethylation is a primary cause or secondary effect of gene expression is unclear. Hypomethylation can be induced by cytidine analogs such as 5-azacytidine.
Selection of HbF-producing erythroid progenitors—cytotoxicity and erythroid regeneration
Adults make small amounts of HbF, restricted to the rare F-cell—erythrocytes with measurable amounts of HbF. Rapid regeneration or expansion of the erythroid marrow induces F-cell production, suggesting that the kinetics of erythroid regeneration determine whether a cell will become an F-cell. Perhaps earlier progenitor cells contain trans-acting factors—fetal erythroid Krüppel-like factor is one example—that favor γ-globin gene expression, while late progenitors express other trans-acting factors favoring β-globin gene expression. Accelerated erythropoiesis increases the chance of premature stem cell commitment and F-cell production. Many cytotoxic drugs can induce HbF expression but cannot directly cause gene hypomethylation—hydroxyurea is one example—providing strong support for the erythroid regeneration mechanism of action of HbF induction.62
Histone deacetylase inhibition
Butyrates appear to directly modulate globin gene expression by binding to transcriptionally active elements.63 Accompanying gene expression is an inhibition of histone deacetylase, histone hyperacetylation, and changes in chromatin structure. Butyrate does not seem cytotoxic and is unlikely to affect HbF levels by inducing erythroid regeneration.
Hemoglobin F-Inducing Agents
5-Azacytidine and butyrate
DeSimone and his coworkers inaugurated the clinical study of hemoglobin “switching” agents. Phlebotomized baboons had a rapid increase in F-cells with a maximum HbF concentration of 2 to 10%, a result explained by the brisk regeneration of erythroid precursors. When 5-azacytidine was added, HbF concentration increased to between 67 and 81%.64 These observations—deemed a result of γ-globin gene hypomethylation—led to clinical trials of 5-azacytidine in patients with sickle cell anemia. That 5-azacytidine might stimulate HbF production via cytotoxicity rather than gene hypomethylation prompted a search for easier to manage “switching” agents.
In sickle cell anemia, initial trials of butyrate given by continuous infusion over a 2- to 3-week interval were inconsistent; newer studies using pulse butyrate treatment are encouraging. When arginine butyrate was given once or twice monthly, 11 of 15 patients with sickle cell anemia responded with a mean increase in HbF from 7% to 21%, a level maintained in some individuals for 1 to 2 years.65 These studies also suggested no cross-resistance between butyrate and hydroxyurea. Pretreatment with hydroxyurea may select a population of erythroid precursors with active γ-globin genes and make them responsive to butyrate. Presently, the use of butyrate remains experimental.
Hydroxyurea in Sickle Cell Anemia
Hydroxyurea arrests DNA synthesis by preventing deoxyribonucleotide formation from ribonucleoside precursors. In anemic primates hydroxyurea increased HbF levels.66 In two patients with sickle cell anemia, hydroxyurea doses of 50 to 100 mg/kg daily in three divided doses increased F-reticulocytes and HbF concentrations. Pilot trials showed increased HbF in treated patients and little short-term toxicity.67
Clinical and hematologic effects
A pivotal efficacy multicenter trial of hydroxyurea in 299 adults with sickle cell anemia showed that hydroxyurea reduced by nearly half the frequency of hospitalizations and the incidence of pain, acute chest syndrome, and blood transfusion.68 In good responders hemolysis and leukocyte counts fell and hemoglobin concentrations increased. HbF increased from a baseline of 5% to about 9% after 2 years of treatment.68 HbF increased to a mean of 18% in the top 25% of HbF responders and to 9% in the next highest 25% but changed little in the lower half of HbF responders.69 These results may not be typical of all patients since the patients in the study had a mean age of about 30 years, had severe disease and were treated to the brink of myelotoxicity.70 Some individuals had improved physical capacity and aerobic cardiovascular fitness.71 A modest improvement in general perceptions of health and social function and recall of pain was found.72 Hydroxyurea was cost effective and clinically beneficial.73
Studies of hydroxyurea in infants, children and adolescents lag behind adult studies in the appraisal of clinical efficacy.74,75,76,77,78 Most reported patients were adolescents or teenagers treated in unblinded pilot studies, but 25 patients, median age 9 years, were treated in a single-blinded crossover study with drug or placebo.77 In all trials, HbF increased from ∼5% before treatment to ∼16% after 6 months to 1 year of treatment.
A trial in 84 children (mean age, ∼10 years) gave results similar to those in adults.79 Sixty-eight patients reached the maximally tolerated dose, and 52 completed 1 year of treatment. About 20% of enrolled patients withdrew from the study, predominantly because of lack of compliance. Baseline HbF of 6.8% increased to 19.8% (range, 3.2% to 32.4%). hematocrit and hemoglobin concentration increased and the leukocyte count fell. These changes, apparent after 6 months of treatment, were sustained at 24 months.80 The increment in HbF was variable; unlike the adults, patients with the highest baseline HbF concentration had the highest HbF levels with treatment, and the decrement in leukocyte count did not predict the HbF increment.
Twenty-nine infants, median age 14 months, were treated with hydroxyurea at a dose of 20 mg/kg for 2 years, escalating to 30 mg/kg thereafter.82 After 2 years, all parents elected to continue treatment and 19 children have completed a median treatment period of 148 weeks. Changes in hemoglobin concentration, hematocrit, HbF and leukocyte count were compared with the changes observed in a historical control group. Hemoglobin increased from 8.5 to 8.9 g/dl (predicted, 8.2 g/dl) MCV increased from 82 to 93 fl (predicted, 88 fl), HbF fell from 21.3 to 19.6% (predicted, 12.3%).81 Functional asplenia was found in 24% of patients before treatment and in 47% after treatment (predicted, 80%). Nine patients were dropped from study for poor compliance or parental refusal to continue; one child died of splenic sequestration. One patient had a transient ischemic attack, one a “mild” stroke, eight had episodes of acute chest syndrome, two had splenic sequestration, and three had episodes of sepsis.82 Growth was normal. Despite impressive levels of HbF, acute complications of sickle cell anemia still occurred in these very young patients. Perhaps functional asplenia is delayed by treatment but further work is needed to document this potentially important result.
Mortality and morbidity
The best data on the complications of hydroxyurea treatment and its effect on morbidity and mortality come from the follow-up of patients in the multicenter trial. By the conclusion of the randomized trial (mean of 28 months of treatment), two hydroxyurea-assigned and six placebo-assigned patients had died, a statistically insignificant difference.83 Mortality was unaffected by age, sex, β-globin gene haplotype, α-globin genotype, F-cell production locus phenotype, HbF level, and crisis rate, but this result may be a consequence of the small numbers of deaths and the selected nature of the patient population.84,85 After 8 years, five strokes had occurred (three fatal) in patients on hydroxyurea and three non-fatal strokes were found in patients on a placebo. New adverse effects have not been found and no patient has developed a neoplasm. After nearly 8 years of follow-up, pulmonary disease was the most common cause of death. In other studies, hydroxyurea alone did not reverse pulmonary hypertension in patients with frequent acute chest episodes although HbF levels were almost 20%; some patients even had progression of their lung disease.86
In 278 patients with HbF measurements after randomization and treatment, cumulative mortality at 8 years was 24% when HbF was < 0.5 g/dL compared with 11% when HbF was > 0.5 g/dL (p = 0.02). Patients taking hydroxyurea during any given quarter during 6 to 8 years of follow-up had a lower mortality rate than patients not taking hydroxyurea. In this analysis there was a 40% reduction in mortality over this observation period (p = 0.04).
We do not know whether hydroxyurea will prevent or even reverse organ damage. After 1 year of treatment, splenic function in children (average age 12 years) did not change.78 Of 10 patients with sickle cell anemia who received hydroxyurea for 21 months and had an increase in HbF from 8 to 17%, recovery of splenic function, ascertained by “pit” counts, Howell-Jolly bodies and 99TC-labeled heat-damaged erythrocyte scans, was present in only one. In a prospective study, some patients had partial return of splenic function and this may be related to HbF levels.87 Splenic regeneration was reported in two adults with sickle cell anemia who had HbF levels of about 30% after hydroxyurea treatment.88
Hydroxyurea does not appear to prevent the cerebrovascular complications of sickle cell anemia. However, in children aged 5 to 15 years, without a history of overt CVA and with more than three painful episodes yearly, hydroxyurea maintained their cognitive performance compared with their sibling controls, whereas performance was noted to deteriorate in untreated patients.89
Prediction of response
Presently, predicting whether a patient will respond to hydroxyurea treatment with an increase in HbF and reduction in vasoocclusive events is not possible. In the multicenter trial, the best HbF-responding patients had the highest initial neutrophil and reticulocyte counts and the largest treatment-associated decrements in these counts. Patients with the greatest reduction in granulocyte, monocyte, and reticulocyte counts also had the largest reduction in painful episodes.70 Individuals with the best HbF response were less likely to have a HbS gene on a Bantu haplotype chromosome.70 Among patients with little change in final HbF level, initial increases in F cells returned to baseline early during treatment. Perhaps, because of marrow “scarring,” some patients are unable to tolerate continual myelosuppressive doses of hydroxyurea.69 In 83 hydroxyurea-treated patients, using 23 different parameters that included age, treatment duration, blood counts and red cell indices, HbF and β-globin gene haplotype, an artificial neural network pattern-recognition analysis predicted the response to hydroxyurea, defined as an increase in HbF to 15%, with ∼85% accuracy.90
In adults, the ability to respond to hydroxyurea may be dependent upon the capacity of the marrow to withstand moderate doses of hydroxyurea with acceptable myelotoxicity. This may be reflected by the baseline reticulocyte and neutrophil counts. Sustained HbF increases during hydroxyurea treatment can occur when bone marrow “reserve” is sufficient to cope with myelotoxicity and the marrow regenerates with erythroblasts capable of substantial HbF synthesis.
How Does Hydroxyurea Work?
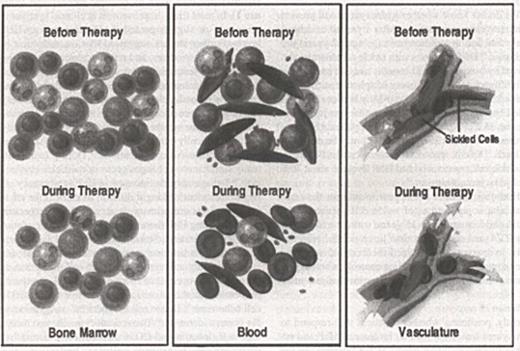
Hydroxyurea may work by multiple mechanisms (Figure 1). In most trials, improvement in clinical symptoms mirrored—or slightly preceded—the increases in HbF levels. Further studies suggested that the reduction in neutrophils, monocytes, and reticulocytes might also be important.70 Neutrophils from patients having a painful episode had increased adherence to cultured endothelial cells.91 By reducing blood neutrophils, hydroxyurea may lessen the chance and severity of vasoocclusive events. Diminishing the numbers of “stress” reticulocytes may be therapeutically beneficial as these cells may also initiate vasoocclusive complications.92 During treatment, many simultaneous changes occur in the sickle erythrocyte.93 Indisputably, the increase in HbF is a primary effect of treatment making it difficult to know if the other myriad effects are primary or secondary. As expected, with increasing HbF there is a reduced rate and extent of HbS polymerization. Sickle cell-endothelial cell adherence decreases before a measured increase in HbF occurs suggesting a direct effect on red cell membrane or the adhesive properties of endothelial cells. Hydroxyurea may make endothelial cells a less attractive site for sickle cell adherence.94 Dense cell numbers fall as erythrocyte K+ content increases.91 Two reticulocyte adhesion receptors, α4β1 integrin and CD36 also fall early during hydroxyurea.95 Improved cellular hydration and deformability are likely to be secondary to increased HbF and may play a role in the reduction of vasoocclusive episodes and the reduced hemolysis accompanying treatment.96
Mechanisms of action of hydroxyurea.
Hydroxyurea acts on the bone marrow and, by its cytotoxic effects, selects a population of erythroblasts that can synthesize increased amounts of HbF. It has no known direct effects on gene expression. Bone marrow cellularity may also be diminished (left). Higher concentrations of HbF reduce the level of HbS polymer and the numbers of deformed, dense, and damaged erythrocytes. Cells with a high HbF content survive longer, attenuating hemolysis and leading to a reduction in reticulocytes. Circulating granulocytes, monocytes, and platelets are diminished (center). Fewer dense, poorly adhesive erythrocytes are less apt to adhere to and perturb the endothelium, reducing the likelihood of vasoocclusion (right).125
Mechanisms of action of hydroxyurea.
Hydroxyurea acts on the bone marrow and, by its cytotoxic effects, selects a population of erythroblasts that can synthesize increased amounts of HbF. It has no known direct effects on gene expression. Bone marrow cellularity may also be diminished (left). Higher concentrations of HbF reduce the level of HbS polymer and the numbers of deformed, dense, and damaged erythrocytes. Cells with a high HbF content survive longer, attenuating hemolysis and leading to a reduction in reticulocytes. Circulating granulocytes, monocytes, and platelets are diminished (center). Fewer dense, poorly adhesive erythrocytes are less apt to adhere to and perturb the endothelium, reducing the likelihood of vasoocclusion (right).125
Hydroxyurea in Other Sickle Hemoglobinopathies
In HbS-β thalassemia, hydroxyurea appears to work as well as in sickle cell anemia.100,101 Pilot studies in adults with HbSC disease indicated that hydroxyurea was associated with sustained increases in mean cell volume (MCV), a fall in absolute reticulocyte counts, “stress” reticulocytes and dense red cells, and a small increase in hematocrit.102 Only a few individuals had increased HbF. Five children with HbSC disease (average age, 13.5 years) and an additional six adults were treated with a median dose of 1000 mg/day of hydroxyurea for 5 to 25 months. Hemoglobin concentration increased by 1 g/dL, MCV increased from 80 to 94 to 103 fl and HbF in children increased from 1.9 to 9.9%.103 Hydroxyurea did not increase the maximal urine concentration of children with HbSC disease.104
Recommendations for Treating Sickle Cell Anemia with Hydroxyurea
An approach to therapy is outlined in Table 1. The availability of 200, 300, and 400 mg hydroxyurea capsules (Droxia™) makes precise dose titration possible. Many different dosing schemes have been recommended, but the sole controlled study gave drug daily and pushed the dose just short of myelotoxicity.105 Experience has suggested that, in most patients, dose escalation to sub-toxic levels is not needed for a satisfactory therapeutic effect. Since MCV rises and usually parallels the increase in HbF when hydroxyurea is used, this inexpensive measurement is a useful surrogate for HbF level that can be serially followed although HbF should be measured periodically.
Indications for hydroxyurea treatment are likely to change as our understanding of its safety and benefits evolve. Several reports suggest hydroxyurea as an alternative to transfusion to prevent new or recurrent stroke, especially when the blood transfusion is not feasible.106,107,108 In 16 children, treatment with hydroxyurea raised HbF to 20% while phlebotomy reduced serum ferritin from 2630 to 636 ng/ml.51 Three new neurological events occurred before HbF was maximized. Other instances of stroke in patients with sickle cell anemia treated with hydroxyurea with “therapeutic” HbF levels have been recorded. HbF levels of 20% do not protect absolutely from other disease events and perhaps stroke is no exception, just a more dramatic example of the shortcomings of a treatment that does not address the entirety of the pathobiology of sickle cell disease. Alternatively, the pathogenesis of cerebral vasculopathy may differ from that of other vasoocclusive complications.
Blood transfusions suppress erythropoiesis, and active erythropoiesis is necessary for hydroxyurea to increase HbF. Although not carefully studied, using hydroxyurea in patients on frequent or regular transfusion programs is probably not efficacious, although some chronically transfused children may have a modest HbF increase when given hydroxyurea.109
Some adult patients will not respond to treatment with an increase in HbF or MCV even when they faithfully take their prescribed medication, although failure to take the medication regularly seems to account for the largest number of “poor responders.”69,110,111 True “non-responders” may number between 10 and 20% of treated patients and be dependent on the dosing regimen, condition of the bone marrow, genetic determinants, and drug metabolism.69
Adverse effects
Long-term effects of hydroxyurea are still not defined. Given the attack rate of leukemia, in the multicenter trial patients there is only power to detect hundredfold increases in the incidence of leukemia or cancer. Hydroxyurea was given to 64 children with cyanotic congenital heart disease for a mean treatment duration of over 5 years without any reports of malignancies.112 Studies of hydroxyurea in patients with myeloproliferative disorders suggest that in this unique setting about 10% of patients treated with hydroxyurea develop acute leukemia.113,114,115,116,117 The author knows of at least three patients with sickle cell disease treated with hydroxyurea who developed leukemia, two after 6 and 8 years of treatment118 (Wilson, personal communication; Smith, personal communication). Cellular changes that may antedate nepotistic transformation, such as increases in chromosome breakage, recombination, or mutations in selected genes, have not been reported in hydroxyurea-treated sickle cell anemia patients.119,120,121 Even with a small risk of leukemogenesis, the benefits of this treatment in seriously ill patients predominate.
Adverse effects on growth and development have not been reported.75 Unknown is whether continued drug exposure starting at a very young age will be especially hazardous—or beneficial. Contraception should be practiced by both women and men receiving hydroxyurea, and the uncertain outcome of an unplanned pregnancy discussed frankly. Pregnancy has been reported in at least 16 women receiving hydroxyurea without adverse outcomes; most had myeloproliferative disorders but six had sickle cell anemia.84,122
Combinations of HbF-inducers and erythropoietin
Conclusions
Hydroxyurea is a valuable adjunct for treating severe sickle cell disease. It must be used carefully with full appreciation of its toxicity and possible long-term adverse effects. Many questions about its use and effects remain unanswered. It is not the final word in the pharmacologic therapy of sickle cell disease but is a promising beginning.