TO THE EDITOR:
Sickle cell disease (SCD) is characterized by repeated episodes of acute pain, also known as vaso-occlusive crises (VOC).1 Activation of sickle hemoglobin (Hb)–containing red blood cells (RBC) promotes multicellular adhesion and occlusion, causing tissue damage secondary to hypoxia, ischemia, and activation of nociceptors.2 Reperfusion promotes chronic inflammation and increased cellular adhesion, further contributing to vaso-occlusion and tissue damage.3,4
Chronic pain (CP) overlaps with nociceptive, neuropathic, and central pain syndromes. CP incidence increases with age, affecting ∼40% of adults with SCD.5,6 The Analgesic, Anesthetic, and Addiction Clinical Trial Translations Innovations Opportunities and Networks-American Pain Society Pain Taxonomy diagnostic criteria for SCD-CP are defined as pain on most days for over 6 months.7 Differentiating between acute pain8 and CP remains challenging. Despite overlapping features, these pain phenotypes likely arise from distinct mechanisms requiring different management strategies.
Increased expression of vascular adhesion biomarkers (VAB), vascular cell adhesion molecule-1 (VCAM), and P-selectin (Psel), promotes leukocyte adhesion and contributes to VOC pathogenesis.9-11 VABs have been studied in SCD during acute VOC but not in CP. We investigated whether VAB differ in individuals with SCD and CP compared to those with SCD and non-CP (NCP).
This 6-month, longitudinal, case-control study evaluated RBC VAB (flow adhesion of whole blood to VCAM-1 [FA-WB-VCAM] and flow adhesion of whole blood to P-selectin [FA-WB-Psel]) in youth with SCD at Children’s National Hospital in Washington, DC. The study was approved by the institutional review board, and all participants were enrolled in the Children’s National Hospital Natural History of SCD Study. Patients with SCD-NCP were age (± 2 years) and genotype matched to patients with SCD-CP. CP inclusion criteria included individuals aged 10 to 21 years and a CP diagnosis per the Analgesic, Anesthetic, and Addiction Clinical Trial Translations Innovations Opportunities and Networks-American Pain Society Pain Taxonomy criteria.7 Exclusion criteria included RBC transfusion within 30 days, transplant failure, pregnancy, active COVID-19, VOC admission within 14 days, or complicated admission within 30 days. Patients on Food and Drug and Administration–approved disease-modifying therapy (DMT), including crizanlizumab, were not excluded from participation. Patient-reported outcomes (PRO) were collected at baseline, at study completion, and during acute pain presentations.12-16 Area Deprivation Index (ADI) were captured.17,18 Data were collected using the REDCap (Research Electronic Data Capture) database.19,20
Standardized microfluidic flow adhesion assays were conducted as described previously.21 Flow adhesion index (fAi) above established critical thresholds for both VCAM and Psel correlated with higher pain scores and increased annualized crises frequency.22 Samples were collected at baseline, 3 months, 6 months (± 6 weeks), and, when applicable, within 24 hours of acute pain presentation. Blood was drawn in sodium citrate tubes, pseudonymized, and shipped overnight to the Functional Fluidics laboratory.
Statistical comparisons were made between patients with CP and NCP and between baseline and VOC state. χ2 test of proportions or a Fisher exact test were used for categorical variables. Depending on the overall distribution, numerical data were assessed using the Student t test or a Wilcoxon rank sum test (SAS version 9.4; Cary, NC). A linear mixed-effects model accounted for repeated measures collected at multiple baseline and VOC time points for each patient. In this model, clinical status (baseline vs VOC) was included as a fixed effect and patient as a random effect to account for within-patient correlations (R, version 4.4.0; RStudio, version 2023.04.21). A P value <.05 was considered statistically significant.
We followed 25 pediatric patients with SCD, including 10 with CP and 15 with NCP. The average age was 16.7 ± 2.8 years, with 64% female and 64% HbSS (Table 1). At study initiation, 70% of CP and 93% of patients with NCP were on hydroxyurea (P = .267) with one-third on combination DMT (P = .999; Table 1). VOC admissions in the preenrollment year were higher in those with CP (P = .009).
Patients with CP had higher acute VOC pain scores (P = .018). Baseline pain (P < .001) and fatigue (P = .003) scores were also higher in the CP cohort and remained so across 3 baseline time points (pain: P < .001 and fatigue: P = .002; Table 1). Patients with CP reported higher centralized pain index scores (P = .013) and a lower average mobility (P = .025), higher pain interference (P = .004), and higher pain intensity (P < .001) in PROMIS (Patient-Reported Outcomes Measurement Information System) domains (supplemental Table 1). Other PRO and ADI scores were not significantly different.
Patients with NCP had a significant increase in white blood cell (WBC) count (P = .038) and decrease in Hb (P = .008) from baseline to acute VOC pain episode. Inflammatory (P = .045) and hemolysis markers (P = .031) were higher during acute pain in NCP, whereas baseline ferritin levels were higher in CP (P = .047) (supplemental Tables 2 and 3). HbF levels were similar (Table 1).
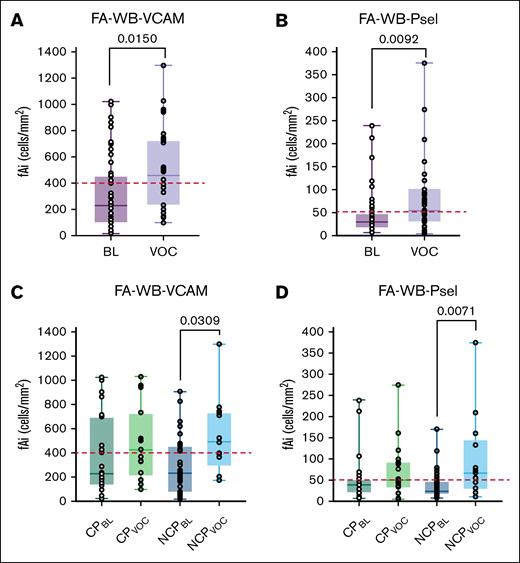
Across all patients, FA-WB-VCAM (P = .0150) and FA-WB-Psel (P = .0092) increased significantly from baseline to VOC (Figure 1A,C). There were no differences in baseline adhesion values (FA-WB-VCAM: P = .3780; FA-WB-Psel: P = .3448) or acute VOC adhesion values between the CP and NCP patients (FA-WB-VCAM: P = .7657; FA-WB-Psel: P = .6365) (Figure 1B,D). There was a significant increase in VABs (FA-WB-VCAM: P = .0309; FA-WB-Psel: P = .0071) in the NCP cohort from baseline to acute VOC (Figure 1B,D). There was no significant change observed in the CP cohort (FA-WB-VCAM: P = .1717; FA-WB-Psel: P = .2953).
FA-WB-VCAM and FA-WB-Psel at BL and during VOC. (A) Across all 25 patients, FA-WB-VCAM increased significantly from BL to VOC (P < .05). (B) No measurable difference in FA-WB-VCAM for patients with CP (n = 10) from BL to VOC, whereas those with NCP (n = 15) displayed a marked increase in VCAM adhesion levels (P < .05). (C) FA-WB-Psel increased significantly from BL to VOC across all 25 patients (P < .01). (D) In CP, there was no measurable change in FA-WB-Psel from BL to VOC in patients with CP (n = 10), whereas those with NCP (n = 15) showed a statistically higher adhesion during VOC compared to BL (P < .01). The red dashed lines represent previously established critical fAi laboratory thresholds: 400 cells per mm2 for FA-WB-VCAM and 50 cells per mm2 for FA-WB-Psel.22 Critical fAi threshold values are associated with a higher probability of a VOC event and poor clinical outcomes. Statistical significance is denoted as P < .05 and P < .01. BL, baseline.
FA-WB-VCAM and FA-WB-Psel at BL and during VOC. (A) Across all 25 patients, FA-WB-VCAM increased significantly from BL to VOC (P < .05). (B) No measurable difference in FA-WB-VCAM for patients with CP (n = 10) from BL to VOC, whereas those with NCP (n = 15) displayed a marked increase in VCAM adhesion levels (P < .05). (C) FA-WB-Psel increased significantly from BL to VOC across all 25 patients (P < .01). (D) In CP, there was no measurable change in FA-WB-Psel from BL to VOC in patients with CP (n = 10), whereas those with NCP (n = 15) showed a statistically higher adhesion during VOC compared to BL (P < .01). The red dashed lines represent previously established critical fAi laboratory thresholds: 400 cells per mm2 for FA-WB-VCAM and 50 cells per mm2 for FA-WB-Psel.22 Critical fAi threshold values are associated with a higher probability of a VOC event and poor clinical outcomes. Statistical significance is denoted as P < .05 and P < .01. BL, baseline.
Our study explores the role of VABs, specifically FA-WB-VCAM and FA-WB-Psel, in pediatric patients with SCD-CP vs NCP. Patients with NCP exhibited significant increases in VAB during acute VOC, whereas patients with CP did not, suggesting distinct adhesion profiles that may illuminate differences in acute pain pathophysiology and inform personalized management.
Under whole blood flow conditions, FA-WB-VCAM reflects RBC adhesiveness to VCAM, while FA-WB-Psel measures WBC adhesiveness to Psel. VCAM is upregulated during endothelial activation and Psel expression by endothelial cells and platelets, a feature of inflammation-related pathologic states.23,24 In our study, patients with NCP demonstrated significant increases in FA-WB-VCAM and FA-WB-Psel during VOC, predisposing to vascular adhesion and inflammation. Patients with CP do not show this inflammatory adhesive response during VOC, suggesting chronic vascular adaptation or other underlying mechanisms. The magnitude of change in VABs from baseline to VOC may be clinically informative.
Patients with CP had more hospitalizations and higher baseline pain and fatigue, indicating greater disease burden. Blunted acute pain score change in CP may signify differences in nociceptive pain processing. PRO confirmed reduced mobility and greater baseline pain interference, fatigue, and anxiety among patients with CP. Socioeconomic disadvantages by ADI did not differ.
During VOC, patients with NCP exhibited increased WBC counts and decreased Hb, compared to those with CP, supporting a hypothesis of chronic inflammation or immune adaptation in CP. Those with CP had higher ferritin levels, potentially reflecting chronic inflammation or prior transfusion burden. Approximately 20% of patients in each cohort were on crizanlizumab, primarily initiated before and continued throughout enrollment. To capture real-world clinical heterogeneity in CP and NCP phenotypes, we included patients receiving DMT, excluding only those receiving routine transfusions.
A limitation of our study is the single-center design and small sample size, which may limit generalizability. Given the exploratory nature of this study and lack of prior fAi data in SCD-CP, we did not conduct power calculations or adjust for multiple comparisons. VAB samples were collected within 24 hours of presentation but not standardized relative to interventions such as IV fluids, crizanlizumab, or analgesia.
To our knowledge, our study is among the first linking RBC adhesion with clinical pain phenotypes in pediatric SCD. Increased FA-WB-VCAM and FA-WB-Psel during VOC in patients with NCP, reflect proadhesive mechanisms driving acute pain and underscore their potential as VABs for SCD pain phenotypes. Future studies should validate these findings in larger cohorts and examine VAB changes in relation to clinical interventions to inform personalized treatment strategies.
Acknowledgments: This work was supported by the American Society of Hematology Hematology Inclusion Pathway Program and, the Ruth L. Kirschstein National Research Service Award Institutional Research Training Grant awarded to the Children’s National Hospital Research Institute Hematology Training Program by the National Heart, Lung, and Blood Institute of the National Institutes of Health (grant 5T32HL110841; O.Y.M.), and by the Children’s National Hospital Sickle Cell Fund (A.D.C.).
Contribution: A.D.C., O.Y.M., and P.C.H. conceptualized the project; A.U.Z., K.R., O.Y.M., R.B., and X.G. wrote the manuscript; A.D.C., D.S.D., and P.C.H. reviewed and edited the manuscript. A.D.C., D.S.D., O.Y.M., and P.C.H. provided programmatic oversight; A.D.C., K.B., and O.Y.M. performed data collection and analysis; A.U.Z., R.B., and X.G. performed biomarker analysis; A.D.C. and X.G. performed statistical analysis; A.D.C., D.S.D., and O.Y.M. delivered care to many of the patients who were enrolled in the study; and all authors contributed to the manuscript and approved the submitted version.
Conflicts-of-interest disclosure: A.D.C. received research funding and consultancy from Novartis, Agios, and bluebird bio; consultancy from Chiesi; and research funding from Novo Nordisk and Pfizer. D.S.D. received consultancy from Pfizer, Novartis, Agios, Novo Nordisk, and Editas. P.C.H. reports employment from and is a shareholder of Functional Fluidics. A.U.Z., R.B., and X.G. report employment from Functional Fluidics. The remaining authors declare no competing financial interests.
Correspondence: Olufunke Y. Martin, Division of Pediatric Hematology/Oncology, Department of Pediatrics, University of Texas Southwestern Medical Center, 5323 Harry Hines Blvd, Dallas, TX 75390; email: olufunke.martin@utsouthwestern.edu.
References
Author notes
Presented in abstract form at the 65th annual meeting of the American Society of Hematology, San Diego, CA, 10 December 2023.
The data sets presented in this article are not readily available due to restrictions provided by the Children’s National Hospital Institutional Review Board. Requests to access data sets should be directed to the corresponding author, Olufunke Y. Martin (olufunke.martin@utsouthwestern.edu).
The full-text version of this article contains a data supplement.