Key Points
PKR activator and GBT021601 lower 2,3-DPG; PKR activator boosts ATP in hypoxia, enhancing sickle-RBC deformability.
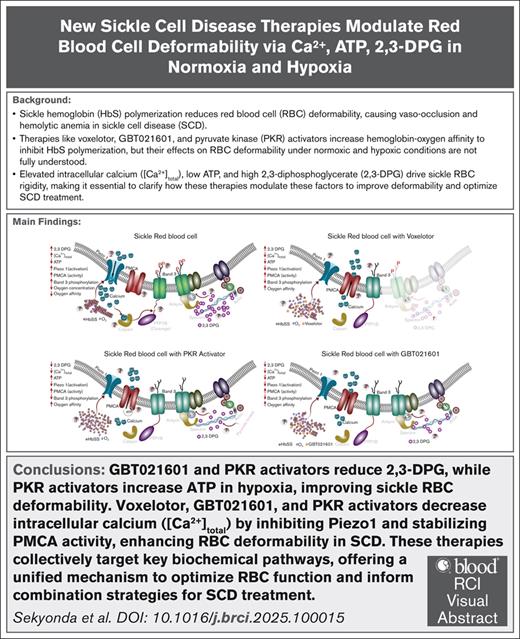
Voxelotor, GBT021601, PKR activator lower [Ca2+]total by inhibiting Piezo1 and modifying clearance pathways, improving RBC deformability.
Visual Abstract
Reduced deformability of red blood cells (RBCs) due to sickle hemoglobin (HbS) polymerization underlies the vaso-occlusive crises and hemolytic anemia that characterize sickle cell disease (SCD). Emerging therapies that increase hemoglobin oxygen affinity (Hb-O2), voxelotor, GBT021601 (second-generation polymerization inhibitor), and pyruvate kinase (PKR) activators have shown clinical benefits. Yet, their specific mechanistic effects on RBC deformability under normoxic and mixed venous hypoxic conditions are not fully understood. We hypothesized that these agents differentially regulate calcium (Ca2+), adenosine triphosphate (ATP), and 2,3-diphosphoglycerate (2,3-DPG), key determinants of deformability, and that hypoxia amplifies their actions. We used targeted biochemical assays to measure total intracellular calcium content ([Ca2+]total), ATP, and 2,3-DPG in sickle (HbSS) and healthy (HbAA) RBCs. Under normoxia, GBT021601 and PKR activators, but not voxelotor, reduced 2,3-DPG. Only PKR activators elevated ATP under hypoxia. All 3 therapies reduced [Ca2+]total in HbSS RBCs by inhibiting Piezo1 activity and modulating Ca2+ clearance pathways, including residual plasma membrane Ca2+-ATPase (PMCA) activity or alternative Ca2+ efflux pathways. Decreased [Ca2+]total coincided with decreases in Band-3 tyrosine phosphorylation in HbSS but not HbAA RBCs, suggesting [Ca2+]total-dependent modulation of membrane-protein interactions. Mechanistically, voxelotor indirectly inhibited Ca2+ entry; GBT021601 reduced 2,3-DPG and potentially modified Ca2+ clearance pathways; and PKR activator paired the 2,3-DPG decrease with hypoxia-specific ATP increases to fuel PMCA activity. Our findings suggest that reduced [Ca2+]total may be a common downstream mechanism by which diverse HbS-targeted and metabolic therapies restore RBC deformability, supporting the exploration of combination strategies targeting complementary pathways in Ca2+ homeostasis and RBC deformability for SCD.
Introduction
Sickle cell disease (SCD) is a genetic disorder characterized by the presence of an abnormal hemoglobin (Hb); hemoglobin S (HbSS). HbSS polymerizes under deoxygenated conditions and deforms red blood cells (RBCs) into rigid and sickled shapes.1,2 Even at normal oxygen (O2) tension (normoxia), residual HbS polymers impose modest membrane tension that activates the mechanosensitive Piezo1 channel, permitting subclinical calcium (Ca2+) influx and subtly reducing deformability.3-5 Under hypoxia, polymerization intensifies, sharply increasing membrane stress, Piezo1 activation, and Ca2+ influx. Elevated intracellular Ca2+ triggers the Gardos channel, driving K+ efflux and water loss.6,7 Consequently, triggering calpain-mediated cytoskeletal breakdown, Band 3 phosphorylation, and thus reducing RBC deformability.8 Impaired RBC deformability leads to severe microvascular occlusion, hemolytic anemia, and painful vaso-occlusive crises characteristic of SCD.2,9 Beyond HbSS polymerization, oxidative stress and chronic inflammation further exacerbate membrane lipid peroxidation and protein oxidation, compounding RBC deformability and microvascular flow.9-11 Understanding the intricacies of RBC deformability has implications for disease management and therapeutic intervention.
Recent therapeutic advances have focused on increasing hemoglobin oxygen (Hb-O2) affinity to prevent HbSS polymerization.12,13 Voxelotor binds the α-chain of HbSS to stabilize its oxygenated (R-state) conformation, reducing HbSS polymerization.14 Similarly, GBT021601, a second-generation HbS polymerization inhibitor, functions through a comparable mechanism to voxelotor.15 Complementing these direct polymer inhibitors, pyruvate kinase (PKR) activators such as mitapivat and etavopivat, which are currently being tested in SCD clinical trials.16,17 PKR activators have multifaceted effects on RBC glycolytic pathways, including increasing the activity of PKR, thereby reducing 2,3-diphosphoglycerate (2,3-DPG) and elevating adenosine triphosphate (ATP) levels.13,18 The therapeutic effect of decreasing 2,3-DPG is to allosterically increase the Hb-O2 affinity, thus delaying the formation of HbS polymers, reducing RBC sickling, and hemolysis, thereby mitigating the anemia and potentially other clinical manifestations of SCD.17,19,20
Despite the clinical benefits, the direct impacts of voxelotor, GBT021601, and PKR activators on RBCs' total intracellular calcium content ([Ca2+]total) homeostasis, cytoskeletal stability, and Band 3 tyrosine phosphorylation, a key regulator of membrane-cytoskeleton interactions,21 remain unstudied. Herein, we systematically evaluated levels of 2,3-DPG, ATP, [Ca2+]total, and Band 3 phosphorylation in SCD (HbSS) and healthy (HbAA) RBCs under normoxic and chemically induced hypoxic (hypoxia) conditions. Furthermore, by using selective inhibitors of the mechanosensitive Piezo1 channel and plasma membrane Ca2+-ATPase (PMCA), we investigated the contribution of Piezo1 activation vs PMCA activity to [Ca2+]total regulation and the resulting impact on microcapillary RBC deformability with treatment.
Using the OcclusionChip, a microcapillary occlusion assay,22-24 we evaluated RBC deformability in HbSS and HbAA samples under normoxic and hypoxic conditions. Unlike ektacytometry, which measures elongation of bulk RBCs under shear stress, the OcclusionChip quantifies the percentage of microchannels blocked by individual cells, providing a single-cell, physiologically relevant assessment that can detect subtle differences in RBC deformability.24 We hypothesized that voxelotor, GBT021601, and PKR activators enhance RBC deformability by differentially regulating intracellular 2,3-DPG, ATP, and [Ca2+]total levels. We observed that all 3 agents significantly reduced [Ca2+]total, potentially via Piezo1 inhibition and modification of Ca2+ clearance pathways, which potentially could involve preserving residual PMCA activity or, alternatively, efflux mechanisms. GBT021601 but not voxelotor lowered 2,3-DPG, whereas PKR activators uniquely increased ATP under hypoxia. These changes in intracellular biomarkers correlated with decreased Band 3 tyrosine phosphorylation, suggesting treatment-specific restoration of sickle RBC integrity via modulation of membrane-protein interactions.
Materials and methods
Blood sample acquisition
Whole blood samples from deidentified healthy controls (HbAA) and individuals with SCD (HbSS) were collected in EDTA tubes at University Hospitals Cleveland Medical Center. Ethical approval (05-14-07C) was granted by University Hospitals Cleveland Medical Center institutional review board, following the Declaration of Helsinki. Samples were stored at 4°C and processed within 10 hours. HbSS was confirmed via high-performance liquid chromatography. Samples from those on voxelotor therapy were excluded. Clinical data for HbSS donor, including samples on hydroxyurea and transfusion medications, are summarized in supplemental Table 1. Additional details on materials and methods are included in the supplemental Information.
Sample preparation
Whole blood samples were processed by centrifugation to isolate RBCs, which were then washed with PBS containing 5 mM glucose and resuspended in a solution with dimethyl sulfoxide to serve as a vehicle control. For drug treatments, RBCs from both control HbAA and SCD patients HbSS were incubated with specific concentrations of PKR activator (HbSS + PKR), voxelotor (HbSS + Vox), or GBT021601 (HbSS + GBT021601) at 37°C for 6 hours on an orbital shaker. Additionally, some samples were pretreated with Grammostola spatulata mechanotoxin 4 (GsMTx4), a Piezo1 inhibitor,25 or sodium orthovanadate, a PMCA inhibitor,26,27 to study their effects in combination with these drugs, creating various experimental groups for subsequent assays (supplemental Notes 1).
Fabrication of the OcclusionChip
As previously described, the OcclusionChip is a functional microcapillary occlusion assay in which RBCs flow through a microfluidic channel packed with a gradient of 20 μm to 4 μm micropillar arrays.23,28 Less deformable RBCs are trapped earlier in the channel, whereas more deformable cells travel further, with side channels mimicking arteriovenous shunts to maintain flow despite upstream occlusion (Figure 1A). The OcclusionChip is cast in polydimethylsiloxane (PDMS) via soft lithography,22,28 then plasma bonded to glass. Before experiments, the chips are functionalized; details are described in supplemental Notes 2.
Vox, PKR activator, and GBT021601 reduce microcapillary occlusion in HbSS RBCs under normoxia and hypoxia. (A) OcclusionChip device used to assess RBC-mediated microcapillary occlusion under normoxic (pO2 ≈ 100 mm Hg) and mixed venous hypoxic (pO2 ≈ 44 mm Hg) conditions. (B) Microcapillary OI comparison between sickle (HbSS) and healthy (HbAA) RBCs under normoxia (HbAA [n = 8] vs HbSS [n = 15]; ∗∗∗P < .001) and hypoxia (HbAA [n = 8] vs HbSS [n = 15]; ∗∗∗P < .001, Mann-Whitney U test). (C) Under normoxia, treatment with Vox, PKR activator, or GBT021601 significantly reduced OI in HbSS RBCs (HbSS vs HbSS + Vox; HbSS vs HbSS + PKR; HbSS vs HbSS + GBT021601; n = 7; ∗P < .05; Wilcoxon test). (D) Similar reductions were observed under hypoxia (HbSS vs HbSS + Vox, n = 12 [∗∗∗P < .001]; HbSS vs HbSS + PKR, n = 12 [∗∗∗P < .001]; HbSS vs HbSS + GBT021601, n = 6 [∗∗∗P < .001]; Wilcoxon test). (E) Summary of mean ± SEM OI for HbAA, HbSS, and treated HbSS RBCs (HbSS + Vox, HbSS + PKR, and HbSS + GBT021601) under normoxic and hypoxic conditions. Error bars are presented as SEM. ns, not significant; SEM, standard error of the mean; Vox, voxelotor.
Vox, PKR activator, and GBT021601 reduce microcapillary occlusion in HbSS RBCs under normoxia and hypoxia. (A) OcclusionChip device used to assess RBC-mediated microcapillary occlusion under normoxic (pO2 ≈ 100 mm Hg) and mixed venous hypoxic (pO2 ≈ 44 mm Hg) conditions. (B) Microcapillary OI comparison between sickle (HbSS) and healthy (HbAA) RBCs under normoxia (HbAA [n = 8] vs HbSS [n = 15]; ∗∗∗P < .001) and hypoxia (HbAA [n = 8] vs HbSS [n = 15]; ∗∗∗P < .001, Mann-Whitney U test). (C) Under normoxia, treatment with Vox, PKR activator, or GBT021601 significantly reduced OI in HbSS RBCs (HbSS vs HbSS + Vox; HbSS vs HbSS + PKR; HbSS vs HbSS + GBT021601; n = 7; ∗P < .05; Wilcoxon test). (D) Similar reductions were observed under hypoxia (HbSS vs HbSS + Vox, n = 12 [∗∗∗P < .001]; HbSS vs HbSS + PKR, n = 12 [∗∗∗P < .001]; HbSS vs HbSS + GBT021601, n = 6 [∗∗∗P < .001]; Wilcoxon test). (E) Summary of mean ± SEM OI for HbAA, HbSS, and treated HbSS RBCs (HbSS + Vox, HbSS + PKR, and HbSS + GBT021601) under normoxic and hypoxic conditions. Error bars are presented as SEM. ns, not significant; SEM, standard error of the mean; Vox, voxelotor.
Occlusion assay under normoxic conditions
RBC samples were introduced into the OcclusionChip under normal oxygen conditions (normoxia; pO2 ≈ 100 mm Hg) for 15 minutes. The chip was imaged to assess the occlusion index (OI), which measures RBC deformability through the percentage of blockages in microchannels, using manual counting and software analysis (Figure 1A).
Occlusion assay under chemically induced hypoxia conditions
The occlusion assay was similar to the normoxia assay but conducted with chemically deoxygenated RBCs to simulate low oxygen mixed venous (pO2 ≈ 44 mm Hg) conditions. Samples were perfused for 12 minutes, and the setup was otherwise identical, focusing on how hypoxia affects RBC occlusion (Figure 1A). Details in supplemental Notes 2.
Measurement of intracellular total calcium content
[Ca2+]total levels in packed RBCs were quantified using atomic absorption spectroscopy29 after treating the cells with hydrochloric acid buffer, with samples processed through multiple centrifugation steps to isolate [Ca2+]total for measurement described in supplemental Notes 3.
Quantitation of RBC Band 3 tyrosine phosphorylation
RBC membranes were prepared as “ghosts” for western blotting to analyze Band 3 protein phosphorylation, a critical determinant of RBC membrane integrity that regulates membrane-cytoskeleton interactions.21 This involved lysing cells, washing the membranes, and then performing protein quantification and blotting. Details are described in supplemental Notes 4 and 5.
Measurement of 2,3-DPG and ATP in RBCs
RBCs were prepared for analysis by adding stable isotope-labeled standards and using methanol for protein precipitation.30 The levels of ATP and 2,3-DPG were then measured using liquid chromatography–mass spectrometry, with detailed standard solutions and equipment calibration described in supplemental Notes 6 and 7.
Statistical analysis
Data from these experiments were analyzed using Minitab software (release 2023, version 21.4), employing various statistical tests like analysis of variance, paired t tests, Wilcoxon or Mann-Whitney U tests, and Shapiro-Wilk test, with significance set at P value <.05.
Results
Microcapillary occlusion in HbSS RBCs is reduced with voxelotor or PKR activator or GBT021601 treatment under normoxia or hypoxia
We employed the OcclusionChip to quantify microcapillary occlusion reported as OI, a measure of RBC deformability22-24 in samples HbAA and those with HbSS under both normoxic and hypoxic conditions (Figure 1A). In HbAA RBCs, OI was low and unchanged between normoxia (1.16% ± 0.67%) and hypoxia (2.34% ± 0.10%; P > .05; Figure 1B). By contrast, untreated HbSS RBCs displayed elevated OI under normoxia (8.19% ± 1.43%; ∗∗∗P < .001 vs HbAA; Figure 1B) that further increased under hypoxia to 38.72% ± 3.46% (∗∗∗P < .001 vs normoxia and ∗∗∗P = .001 vs HbAA; Figure 1B,E). Under normoxia, HbSS + Vox, HbSS + PKR, and HbSS + GBT021601 each markedly reduced OI in HbSS RBCs (from 8.19% ± 1.43% to 8.41% ± 1.67%, 7.69% ± 2.17%, and 9.11% ± 1.88%, respectively; ∗P < .05 vs untreated; Figure 1C,E). Under hypoxia, these agents similarly lowered OI from 38.72% ± 3.46% to 18.49% ± 2.78% (HbSS + Vox), 23.94% ± 3.28% (HbSS + PKR), and 19.93% ± 3.58% (HbSS + GBT021601; ∗∗P < .01 and ∗∗∗P < .001; Figure 1D-E). Voxelotor reduced OI by approximately 52.23%, PKR activators by 38.96%, and GBT021601 by 48.50%. These findings suggest that (1) HbSS RBCs are far less deformable than HbAA RBCs and (2) treatment with voxelotor, PKR activators, or GBT021601 restores deformability, lowering occlusion to HbAA RBC levels in both normoxia and hypoxia.
PKR activators and GBT021601 significantly reduce 2,3-DPG in HbSS RBCs, whereas voxelotor does not affect 2,3-DPG under either normoxia or hypoxia
We quantified ATP and 2,3-DPG in RBCs, since high 2,3-DPG and low ATP are correlated with enhanced membrane dynamics and reduced deformability.31-33 Here, we found that under normoxia, HbAA RBCs exhibited higher ATP (190.5 ± 18.6 μg/mL) than HbSS RBCs (138.6 ± 13.9 μg/mL; ∗P < .05; Figure 2A) HbSS + Vox and HbSS + PKR did not alter ATP levels in HbSS RBCs (137.6 ± 14.3 and 129.1 ± 9.6 μg/mL, respectively; P > .05), whereas HbSS + GBT021601 modestly decreased ATP (101.0 ± 12.6 μg/mL; P = .058; Figure 2A). Normoxic 2,3-DPG was lower in HbAA (111.6 ± 11.6 μg/mL) compared to HbSS RBCs (157.6 ± 35.4 μg/mL; ∗P < .05; Figure 2B). PKR activators (86.0 ± 18.9 μg/mL) and GBT021601 (64.3 ± 10.9 μg/mL) significantly reduced 2,3-DPG compared to untreated HbSS (157.6 ± 35.4 μg/mL; ∗∗P < .01; Figure 2B), whereas voxelotor (139.5 ± 32.2 μg/mL) had no effect (P > .05; Figure 2B).
PKR activator or GBT021601 reduces 2,3-DPG levels in HbSS RBCs and modulates ATP under normoxia and hypoxia. (A) Under normoxia, HbAA RBCs exhibited higher ATP levels compared to HbSS RBCs (HbAA [n = 5] vs HbSS [n = 27]; ∗P < .05; Mann-Whitney U test). ATP levels in untreated HbSS RBCs were unaffected by treatment with Vox, PKR activator, or GBT021601 (ns, P > .05; Wilcoxon test). (B) Normoxic 2,3-DPG levels were lower in HbAA RBCs than in HbSS RBCs (HbAA, n = 5 vs HbSS, n = 27; ∗P < .05, Mann-Whitney U test). PKR activator and GBT021601 reduced 2,3-DPG levels in HbSS RBCs (HbSS vs HbSS + PKR [n = 27; ∗∗P < .01; Wilcoxon test]; HbSS vs HbSS + GBT021601 [n = 5; ∗∗P < .01; Wilcoxon test]), whereas Vox had no effect (ns, P > .05; Wilcoxon test). (C) Under hypoxia, HbAA RBCs retained higher ATP levels than HbSS RBCs (HbAA [n = 5] vs HbSS [n = 10]; ∗P < .05; Mann-Whitney U test). PKR treatment increased ATP levels (HbSS vs HbSS + PKR; n = 10; ∗∗P < .01; Wilcoxon test), whereas Vox and GBT021601 had no effect (ns; P > .05; Wilcoxon test). (D) Similar to normoxia, hypoxic 2,3-DPG levels were lower in HbAA RBCs compared to HbSS RBCs (HbAA [n = 5] vs HbSS [n = 10]; ∗P < .05; Mann-Whitney U test). PKR activator and GBT021601 reduced 2,3-DPG levels (HbSS vs HbSS + PKR; ∗P < .05; HbSS vs HbSS + GBT021601; ∗P < .05; Wilcoxon test), whereas Vox had no effect (ns, P > .05; Wilcoxon test). Error bars are presented as SEM. ns, not significant.
PKR activator or GBT021601 reduces 2,3-DPG levels in HbSS RBCs and modulates ATP under normoxia and hypoxia. (A) Under normoxia, HbAA RBCs exhibited higher ATP levels compared to HbSS RBCs (HbAA [n = 5] vs HbSS [n = 27]; ∗P < .05; Mann-Whitney U test). ATP levels in untreated HbSS RBCs were unaffected by treatment with Vox, PKR activator, or GBT021601 (ns, P > .05; Wilcoxon test). (B) Normoxic 2,3-DPG levels were lower in HbAA RBCs than in HbSS RBCs (HbAA, n = 5 vs HbSS, n = 27; ∗P < .05, Mann-Whitney U test). PKR activator and GBT021601 reduced 2,3-DPG levels in HbSS RBCs (HbSS vs HbSS + PKR [n = 27; ∗∗P < .01; Wilcoxon test]; HbSS vs HbSS + GBT021601 [n = 5; ∗∗P < .01; Wilcoxon test]), whereas Vox had no effect (ns, P > .05; Wilcoxon test). (C) Under hypoxia, HbAA RBCs retained higher ATP levels than HbSS RBCs (HbAA [n = 5] vs HbSS [n = 10]; ∗P < .05; Mann-Whitney U test). PKR treatment increased ATP levels (HbSS vs HbSS + PKR; n = 10; ∗∗P < .01; Wilcoxon test), whereas Vox and GBT021601 had no effect (ns; P > .05; Wilcoxon test). (D) Similar to normoxia, hypoxic 2,3-DPG levels were lower in HbAA RBCs compared to HbSS RBCs (HbAA [n = 5] vs HbSS [n = 10]; ∗P < .05; Mann-Whitney U test). PKR activator and GBT021601 reduced 2,3-DPG levels (HbSS vs HbSS + PKR; ∗P < .05; HbSS vs HbSS + GBT021601; ∗P < .05; Wilcoxon test), whereas Vox had no effect (ns, P > .05; Wilcoxon test). Error bars are presented as SEM. ns, not significant.
Under hypoxia, HbAA ATP (130.5 ± 9.6 μg/mL) remained significantly higher than HbSS ATP (78.6 ± 9.9 μg/mL; ∗P < .05; Figure 2C). ATP levels of untreated HbSS RBCs (78.6 ± 9.9 μg/mL) did not differ from either HbSS + Vox (70.8 ± 8.26 μg/mL) or HbSS + GBT021601 (55.8 ± 3.31 μg/mL; ∗P > .05 for both comparisons; Figure 2C). HbSS + Vox and HbSS + GBT021601 did not significantly alter hypoxic ATP levels (70.8 ± 8.3 and 55.8 ± 3.3 μg/mL, respectively; P > .05), whereas HbSS + PKR activators increased ATP levels to 97.0 ± 16.3 μg/mL (∗∗P < .01; Figure 2C). Hypoxic 2,3-DPG was lower in HbAA (147.0 ± 8.6 μg/mL) than in HbSS RBCs (210.6 ± 19.2 μg/mL; ∗P < .05; Figure 2D). HbSS + PKR (120.1 ± 10.6 μg/mL) and HbSS + GBT021601 (115.0 ± 18.9 μg/mL) significantly reduced hypoxic 2,3-DPG vs untreated (210.6 ± 19.2 μg/mL; ∗P < .05), whereas voxelotor (190.5 ± 17.2 μg/mL) remained the same (P > .05; Figure 2D). These findings indicate that voxelotor and GBT021601 do not affect ATP, but PKR activators increased ATP only under hypoxia. Both PKR activators and GBT021601 consistently lower 2,3-DPG in HbSS RBCs, whereas voxelotor has no impact on 2,3-DPG in either normoxia or hypoxia.
Intracellular [Ca2+]total in packed HbSS RBCs is reduced by voxelotor, PKR activators, and GBT021601 via inhibition of Piezo1 and modulating alternative Ca2+ clearance pathways under normoxic and hypoxic
Given the pivotal role of Ca2+ in RBC deformability, we hypothesized that voxelotor, PKR activators, and GBT021601 would modulate intracellular [Ca2+]total levels. Elevated Ca2+ activates calpain and calmodulin, which remodel cytoskeletal proteins to influence the membrane.5,7 Pathological Ca2+ overload contributes to RBC dehydration and hypoxia-induced sickling. Thus, we assessed how these drugs, by altering [Ca2+]total dynamics, impact RBC occlusion under normoxia and hypoxia.
Under normoxia, [Ca2+]total levels in untreated HbAA RBCs (80.6 ± 21.9 μmol/L) were comparable to that in RBCs treated with voxelotor (87.2 ± 14.9 μmol/L) or PKR activators (99.8 ± 23.5 7 μmol/L; P > .05 for all; Figure 3A). In contrast, HbSS RBCs showed elevated [Ca2+]total (145.20 ± 10.85 μmol/L), which was significantly reduced by voxelotor (102.68 ± 7.69 μmol/L), PKR activators (109.23 ± 9.01 μmol/L), or GBT021601 (99.92 ± 13.58 μmol/L; ∗∗∗P < .001 for all; Figure 3B).
Vox, PKR activator, and GBT021601 reduced [Ca2+]total levels and improved HbSS RBC occlusion by Piezo1 inhibition and enhancing residual PMCA activity under normoxic and hypoxic conditions. (A) In HbAA RBCs, Vox and PKR activator did not change the basal intracellular [Ca2+]total levels (n = 5; ns, P > .05; Wilcoxon test). (B) Under normoxia, [Ca2+]total levels were significantly reduced in HbSS RBCs treated with Vox (HbSS vs HbSS + Vox; n = 21), PKR activator (HbSS vs HbSS + PKR; n = 21), or GBT021601 (HbSS vs HbSS + GBT021601; n = 5), ∗∗∗P < .001, t test for all. Relatively greater reductions were observed under hypoxia (HbSS + Vox, n = 6; HbSS + PKR, n = 6; HbSS + GBT021601, n = 6; ∗∗∗P < .001; Wilcoxon test for all). (C) Piezo1 blockade with GsMTx4 reduced intracellular [Ca2+]total levels under normoxia and hypoxia, but did not differ significantly with cotreatment with Vox, PKR activator, and GBT021601 (∗∗∗P < .001; n = 5; Wilcoxon test for all). (D) PMCA inhibition with vanadate increases [Ca2+]total levels under both normoxia and hypoxia; cotreatment with Vox, PKR activator, and GBT021601 significantly lowered the [Ca2+]total levels despite PMCA blockade (∗∗∗P < .001; n = 5; Wilcoxon test for all). (E) Similar to [Ca2+]total levels, OI under GsMTx4 alone was reduced compared with untreated HbSS RBCs (∗P > .05; n = 5; Wilcoxon test), and did not differ significantly with cotreatment with Vox, PKR activator, and GBT021601 (ns, P > .05; n = 7; t test for all). (F) Vanadate increased OI under both normoxia (n = 5; ∗P < .05; Wilcoxon test) and hypoxia (n = 5; ∗∗∗P < .001; Wilcoxon test). Cotreatment with Vox, PKR activator, or GBT021601 significantly lowered the OI (n = 5; ∗P < .05, ∗∗P < .01, ∗P < .05; Wilcoxon test). Error bars are presented as mean ± SEM. Gs, Grammostola spatulata mechanotoxin 4.
Vox, PKR activator, and GBT021601 reduced [Ca2+]total levels and improved HbSS RBC occlusion by Piezo1 inhibition and enhancing residual PMCA activity under normoxic and hypoxic conditions. (A) In HbAA RBCs, Vox and PKR activator did not change the basal intracellular [Ca2+]total levels (n = 5; ns, P > .05; Wilcoxon test). (B) Under normoxia, [Ca2+]total levels were significantly reduced in HbSS RBCs treated with Vox (HbSS vs HbSS + Vox; n = 21), PKR activator (HbSS vs HbSS + PKR; n = 21), or GBT021601 (HbSS vs HbSS + GBT021601; n = 5), ∗∗∗P < .001, t test for all. Relatively greater reductions were observed under hypoxia (HbSS + Vox, n = 6; HbSS + PKR, n = 6; HbSS + GBT021601, n = 6; ∗∗∗P < .001; Wilcoxon test for all). (C) Piezo1 blockade with GsMTx4 reduced intracellular [Ca2+]total levels under normoxia and hypoxia, but did not differ significantly with cotreatment with Vox, PKR activator, and GBT021601 (∗∗∗P < .001; n = 5; Wilcoxon test for all). (D) PMCA inhibition with vanadate increases [Ca2+]total levels under both normoxia and hypoxia; cotreatment with Vox, PKR activator, and GBT021601 significantly lowered the [Ca2+]total levels despite PMCA blockade (∗∗∗P < .001; n = 5; Wilcoxon test for all). (E) Similar to [Ca2+]total levels, OI under GsMTx4 alone was reduced compared with untreated HbSS RBCs (∗P > .05; n = 5; Wilcoxon test), and did not differ significantly with cotreatment with Vox, PKR activator, and GBT021601 (ns, P > .05; n = 7; t test for all). (F) Vanadate increased OI under both normoxia (n = 5; ∗P < .05; Wilcoxon test) and hypoxia (n = 5; ∗∗∗P < .001; Wilcoxon test). Cotreatment with Vox, PKR activator, or GBT021601 significantly lowered the OI (n = 5; ∗P < .05, ∗∗P < .01, ∗P < .05; Wilcoxon test). Error bars are presented as mean ± SEM. Gs, Grammostola spatulata mechanotoxin 4.
Under hypoxia, similar drug-induced reductions occurred: voxelotor (102.2 ± 13.6 μmol/L), PKR activators (137.8 ± 15.7 μmol/L), and GBT021601 (125.0 ± 13.8 μmol/L) all lowered [Ca2+]total relative to untreated HbSS RBCs (222.6 ± 23.6 μmol/L; ∗∗∗P < .001; for all; Figure 3B). To probe the role of Piezo1, we treated HbSS RBCs with the Piezo1 inhibitor GsMTx4. Under normoxia, GsMTx4 alone (105.0 ± 8.2 μmol/L) yielded [Ca2+]total levels comparable to cotreatments with voxelotor (86.7 ± 10.2 μmol/L), PKR activators (91.8 ± 8.9 μmol/L), or GBT021601 (85.9 ± 9.2 μmol/L; P > .05; Figure 3C) Similarly, under hypoxia, GsMTx4 (116.0 ± 18.9 μmol/L) likewise matched cotreatments (voxelotor, 97.0 ± 17.9 μmol/L; PKR activators, 94.8 ± 18.3 μmol/L; GBT021601, 80.0 ± 14.7 μmol/L; P > .05 for all; Figure 3C). We next inhibited PMCA with sodium orthovanadate to isolate its contribution to [Ca2+]total with drug treatment. Under normoxia, vanadate increased [Ca2+]total (186.5 ± 14.9 μmol/L), which was then reduced by cotreatment with voxelotor (121.8 ± 17.7 μmol/L), PKR activators (125.2 ± 12.5 μmol/L), or GBT021601 (123.8 ± 17.9 μmol/L; ∗∗∗P < .001 for all; Figure 3D). Under hypoxia, vanadate further elevated [Ca2+]total (238.1 ± 29.7 μmol/L), but each drug reduced this (voxelotor, 149.0 ± 18.6 μmol/L; PKR activators, 122.0 ± 15.0 μmol/L; GBT021601, 148.4 ± 18.5 μmol/L; ∗∗∗P < .001 for all; Figure 3D). Finally, we measured the OI after GsMTx4 or vanadate treatments (Figure 3E-F). GsMTx4 under normoxia reduced OI (18.0% ± 1.7% vs 25.3% ± 3.3%; ∗P < .05), but cotreatments did not further improve it (voxelotor [15% ± 0.94%], PKR activators [16.53% ± 0.89%], or GBT021601 [16.307% ± 1.5%]; P > .05 for all; Figure 3E). Under hypoxia, GsMTx4 also decreased occlusion (55.3% ± 6.3% vs 63.5% ± 7.7%; ∗∗P < .01) with no added benefit from cotreatments and voxelotor (46.9% ± 7.6%), PKR activators (46.2% ± 5.68%), or GBT021601 (38.54% ± 6.37; P > .05 for all; Figure 3E). Vanadate increased occlusion under normoxia (18.7% ± 1.4% vs 14.3% ± 1.4%; ∗P < .05) but was reduced by cotreatments voxelotor (11.6% ± 1.9%), PKR activators (13.16% ± 1.81%), or GBT021601 (11.34% ± 1.4%; ∗P < .05 for all; Figure 3F). Under hypoxia, vanadate also increased occlusion (62.4% ± 1.3% vs 32.0% ± 8.3%; ∗∗∗P < .001). Cotreatments significantly reduced occlusion in both conditions: voxelotor (48.01% ± 6.3%), PKR activators (37.31% ± 4.33%), or GBT021601 (47.96% ± 6.3%; ∗P < .05, ∗∗P < .01, ∗P < .05, respectively; Figure 3F). These results indicate that (1) these agents selectively lower elevated [Ca2+]total in HbSS RBCs without affecting HbAA RBCs, (2) Piezo1 inhibition induces further [Ca2+]total reduction, suggesting a shared mechanism via Piezo1, (3) PMCA inhibition elevates [Ca2+]total and occlusion, and (4) Cotreatment with voxelotor, PKR activators, or GBT021601 attenuates PMCA inhibitor induced [Ca2+]total overload and occlusion, suggesting modification of alternative Ca2+ clearance pathways.
Treatments of HbSS RBCs with voxelotor, PKR activators, or GBT021601 reduce Band 3 tyrosine phosphorylation levels under normoxic and hypoxic conditions
Previous studies have reported elevated tyrosine phosphorylation of Band 3 in HbSS RBCs.8,21 To evaluate the impact of voxelotor, PKR activators, and GBT021601 on membrane protein phosphorylation, we quantified Band 3 tyrosine phosphorylation levels under normoxic and hypoxic conditions. Under normoxia, phosphorylation levels in untreated HbAA RBCs (99.9 ± 6.0) were comparable to those in HbAA RBCs treated with voxelotor (100.12 ± 7.44) or PKR activators (96.86 ± 5.78) with no significant differences (P > .05 for both; Figure 4A-B).
Vox, PKR activator, and GBT021601 reduce Band 3 tyrosine phosphorylation in HbSS RBCs under normoxia and hypoxia. (A) A total of 20 μg of total protein was loaded per lane for all immunoblots. Representative anti–Band 3 immunoblots for untreated and treated HbAA and HbSS RBCs (Vox, PKR activator, GBT021601). (B) Band 3 tyrosine phosphorylation levels in HbAA RBCs were unaffected by treatment with Vox or PKR activator (HbAA vs HbAA + Vox and HbAA + PKR; n = 7; ns, P > .05; Wilcoxon test). (C) Under normoxia, Band 3 tyrosine phosphorylation levels were significantly reduced in HbSS RBCs treated with Vox (HbSS vs HbSS + Vox; n = 22; ∗∗∗P < .001; t test), PKR activator (HbSS vs HbSS + PKR; n = 22; ∗∗∗P < .001; t test), and GBT021601 (HbSS vs HbSS + GBT021601; n = 5; ∗P < .05; t test). (D) Similar reductions were observed under hypoxia (HbSS + Vox [n = 10; ∗∗P < .01]; HbSS + PKR [n = 10; ∗∗P < .01]; HbSS + GBT021601 [n = 10; ∗∗P < .01; t test]). Error bars are presented as mean ± SEM.
Vox, PKR activator, and GBT021601 reduce Band 3 tyrosine phosphorylation in HbSS RBCs under normoxia and hypoxia. (A) A total of 20 μg of total protein was loaded per lane for all immunoblots. Representative anti–Band 3 immunoblots for untreated and treated HbAA and HbSS RBCs (Vox, PKR activator, GBT021601). (B) Band 3 tyrosine phosphorylation levels in HbAA RBCs were unaffected by treatment with Vox or PKR activator (HbAA vs HbAA + Vox and HbAA + PKR; n = 7; ns, P > .05; Wilcoxon test). (C) Under normoxia, Band 3 tyrosine phosphorylation levels were significantly reduced in HbSS RBCs treated with Vox (HbSS vs HbSS + Vox; n = 22; ∗∗∗P < .001; t test), PKR activator (HbSS vs HbSS + PKR; n = 22; ∗∗∗P < .001; t test), and GBT021601 (HbSS vs HbSS + GBT021601; n = 5; ∗P < .05; t test). (D) Similar reductions were observed under hypoxia (HbSS + Vox [n = 10; ∗∗P < .01]; HbSS + PKR [n = 10; ∗∗P < .01]; HbSS + GBT021601 [n = 10; ∗∗P < .01; t test]). Error bars are presented as mean ± SEM.
In contrast, HbSS RBCs exhibited significantly elevated Band 3 phosphorylation levels (110.28 ± 4.38), which were markedly reduced following treatment with voxelotor (75.39 ± 4.94), PKR activators (69.83 ± 6.23), or GBT021601 (75.98 ± 12.75) with respective statistical significance (∗∗∗P < .001, ∗∗∗P < . 001, ∗P < .05, respectively; Figure 4A,C). Under hypoxia, Band 3 phosphorylation levels in untreated HbSS RBCs (225 ± 7.38) increased further, but voxelotor (162.4 ± 4.56), PKR activators (125.6 ± 7.89), and GBT021601 (145.89 ± 3.45) significantly reduced phosphorylation levels (∗∗P < .01 for all; Figure 4A,D). These findings demonstrate that voxelotor, PKR activators, and GBT021601 selectively reduce Band 3 tyrosine phosphorylation in HbSS RBCs without affecting HbAA RBCs. The pattern mirrors prior observations of drug-induced normalization of intracellular [Ca2+]total levels and is consistent with a potential link between [Ca2+]total dynamics and Band 3 phosphorylation in SCD.8
Discussion
Recent developments in disease-modifying therapies for SCD have significantly expanded the therapeutic armamentarium.34,35 The use of Hb-O2 affinity–modifying drugs, such as voxelotor, GBT021601, and activation of erythrocyte PKR, presents an intriguing avenue for treatment. These approaches increase Hb-O2 affinity and limit HbSS polymerization.31-34 However, the mechanisms by which increased Hb-O2 affinity improves RBC deformability, particularly via surrogate markers of cytoskeletal stability under normoxic and hypoxic conditions, remain insufficiently explored.
In this study, we leveraged our understanding of HbSS RBC deformability under normoxic (pO2 ≈ 100 mm Hg) and mixed venous hypoxic (pO2 ≈ 44 mm Hg) conditions.24,36 Using the OcclusionChip microcapillary assay to measure microcapillary occlusion OI in RBCs from HbAA and HbSS donors under normoxia and chemically induced hypoxia.22,23 We showed that untreated HbSS RBCs exhibited dramatically elevated OI under normoxia (∼8% vs ∼1% in HbAA; ∗∗∗P < .001) that worsened under hypoxia (∼39%; ∗∗∗P < .001 vs normoxia and HbAA). These findings align with evidence that a measurable residual deoxygenated-HbS persists and forms small polymer fibers under normoxia, even at arterial oxygen saturations above 90% to 95%,37-39 generating sufficient membrane tension to activate Piezo1 channel, elicit subclinical Ca2+ influx, and subtly impair deformability.40,41 Under hypoxic stress, HbS polymer formation mechanically deforms the RBC membrane to a far greater extent than under normoxia, an effect reflected in our data by markedly increased OI under hypoxia. This mechanical deformation directly activates the mechanosensitive Piezo1 Ca2+ influx channel, leading to accumulation of [Ca2+]total that exceeds the capacity of PMCA Ca2+ efflux, and thus triggers the Ca2+-sensitive Gardos (K+) channel.5,42 The resulting K+ efflux and osmotic water loss dehydrate the cell, further stiffening the membrane and exacerbating sickling, thus tightly linking hypoxia-induced polymerization to disrupted Ca2+ homeostasis and the dehydration pathway in SCD. The cascade of intracellular Ca2+ overload, calpain-mediated cytoskeletal breakdown, and Band 3 phosphorylation culminates in which turn, reduces RBC deformability.8,41
All 3, voxelotor, GBT021601, or PKR activator treatments, significantly reduced HbSS OI toward HbAA levels in both normoxic and hypoxic conditions but via distinct molecular routes, demonstrating restoration of RBC deformability. Voxelotor and GBT021601 stabilize the oxygenated (R-state) form of HbS via Schiff-base interactions, increasing Hb-O2 affinity, reducing the deoxy-HbS pool under normoxia, and preventing polymer formation under hypoxia.43,44 PKR activators mitigate sickling through distinct but complementary mechanisms: diverting from the Rapoport-Leubering shunt and thereby, lowering 2,3-DPG, which indirectly raises Hb-O2 affinity, and modulates ATP levels.35,45
HbSS RBCs have been reported to exhibit elevated 2,3-DPG, reduced ATP, [Ca2+]total, and elevated Band 3 tyrosine phosphorylation levels21,46,47 (Figures 5A and 6A). Elevated 2,3-DPG reduces Hb-O2 affinity, thereby promoting HbS polymerization.48 Additionally, increased 2,3-DPG interacts with spectrin and other cytoskeletal components, enhancing membrane viscoelasticity, thus contributing to impaired erythrocyte deformability.49 Reduced ATP levels adversely affect the activity of the PMCA pump, leading to elevated intracellular calcium, which triggers downstream phosphorylation of membrane proteins such as Band 3, destabilizing membrane-cytoskeletal interactions.8,21,50 We systematically examined intracellular ATP, 2,3-DPG, [Ca2+]total, and Band 3 tyrosine phosphorylation under both normoxic and hypoxic conditions. Voxelotor did not alter ATP or 2,3-DPG in HbSS RBCs. GBT021601 did not alter ATP but significantly reduced 2,3-DPG under normoxia and hypoxia. Interestingly, despite their shared R-state stabilization of HbS, the divergent effects of GBT021601 and voxelotor on 2,3-DPG underscore distinct interactions with RBC metabolism.15,44 GBT021601’s potential to reduce 2,3-DPG suggests it also alters the Rapoport-Leubering shunt. Preclinical studies have reported that GBT021601 achieves higher Hb occupancy at lower doses than voxelotor, which can translate into more potent inhibition of polymer formation and a concomitant reduction in 2,3-DPG that may further enhance Hb-O2 affinity and membrane stability.15 PKR activators lowered 2,3-DPG in both conditions and increased ATP only under hypoxia. Previous reports show ATP increases with PKR activation under normoxia in vitro17 and murine SCD models16 after 10 hours, which was not significant in 6 hours of incubation in our normoxic study. This dichotomy can likely be explained that the ATP increase may manifest after prolonged treatment exposure under normoxia, whereas a decrease in 2,3-DPG reflects the most immediate effect of PKR activators.
Vox, PKR activator, and GBT021601 improve HbSS RBC deformability via distinct pathways under normoxia. (A) In untreated HbSS cells, residual deoxy-HbS polymers are present, even at relatively high levels of oxygenation, and they are exacerbated by elevated 2,3-DPG, which imposes membrane tension that activates Piezo1 and elicits influx. Concurrent ATP depletion impairs PMCA, leading to [Ca2+]total overload, calpain activation, and Band 3 phosphorylation. Phosphorylated Band 3 dissociates from the spectrin-actin-protein 4.1 network, disrupting membrane cytoskeleton cohesion and thereby reducing deformability. (B) Vox covalently stabilizes HbSS in its R-state, reducing deoxy-HbS polymer formation without altering ATP or 2,3-DPG. Lower membrane tension limits Piezo1 opening and influx, normalizing [Ca2+]total. Reduced [Ca2+]total results in Band 3 dephosphorylation, maintains PMCA microdomains, and preserves cytoskeletal integrity, thereby improving deformability. (C) PKR activators enhance PKR activity to lower 2,3-DPG. Reduced 2,3-DPG increases Hb-O2 affinity, suppresses polymerization, and decreases membrane stress–induced Piezo1 activation. By preserving residual PMCA function and restoring Ca2+ homeostasis, PKR activators reduce Band 3 tyrosine phosphorylation and stabilize the spectrin-actin-protein 4.1 cytoskeletal complex, markedly improving RBC membrane integrity and deformability. (D) GBT021601 combines R-state stabilization with robust 2,3-DPG lowering. The dual effect suppresses HbS polymerization, reduces membrane tension, inhibits Piezo1, and maintains residual PMCA activity. The resulting [Ca2+]total reduction limits Band 3 phosphorylation, stabilizes the membrane-cytoskeleton network, and markedly enhances RBC deformability.
Vox, PKR activator, and GBT021601 improve HbSS RBC deformability via distinct pathways under normoxia. (A) In untreated HbSS cells, residual deoxy-HbS polymers are present, even at relatively high levels of oxygenation, and they are exacerbated by elevated 2,3-DPG, which imposes membrane tension that activates Piezo1 and elicits influx. Concurrent ATP depletion impairs PMCA, leading to [Ca2+]total overload, calpain activation, and Band 3 phosphorylation. Phosphorylated Band 3 dissociates from the spectrin-actin-protein 4.1 network, disrupting membrane cytoskeleton cohesion and thereby reducing deformability. (B) Vox covalently stabilizes HbSS in its R-state, reducing deoxy-HbS polymer formation without altering ATP or 2,3-DPG. Lower membrane tension limits Piezo1 opening and influx, normalizing [Ca2+]total. Reduced [Ca2+]total results in Band 3 dephosphorylation, maintains PMCA microdomains, and preserves cytoskeletal integrity, thereby improving deformability. (C) PKR activators enhance PKR activity to lower 2,3-DPG. Reduced 2,3-DPG increases Hb-O2 affinity, suppresses polymerization, and decreases membrane stress–induced Piezo1 activation. By preserving residual PMCA function and restoring Ca2+ homeostasis, PKR activators reduce Band 3 tyrosine phosphorylation and stabilize the spectrin-actin-protein 4.1 cytoskeletal complex, markedly improving RBC membrane integrity and deformability. (D) GBT021601 combines R-state stabilization with robust 2,3-DPG lowering. The dual effect suppresses HbS polymerization, reduces membrane tension, inhibits Piezo1, and maintains residual PMCA activity. The resulting [Ca2+]total reduction limits Band 3 phosphorylation, stabilizes the membrane-cytoskeleton network, and markedly enhances RBC deformability.
Vox, PKR activator, and GBT021601 enhance HbSS RBC deformability under hypoxia through distinct mechanisms. (A) In untreated HbSS cells, increased deoxygenation drives extensive HbSS polymerization, membrane deformation, and stretching; this elevated tension robustly activates Piezo1 channels, leading to marked influx, ATP depletion, and secondary reductions in 2,3-DPG. The resultant [Ca2+]total overload triggers Band 3 phosphorylation, dissociation from the spectrin-actin-protein 4.1 network, and severe loss of deformability. (B) Vox increases Hb-O2 affinity under hypoxia, preventing residual polymer formation and membrane stress. Reduced Piezo1 activation limits influx, whereas improved membrane tension maintains residual PMCA activity. Together, these actions lower intracellular [Ca2+]total, reduce Band 3 phosphorylation, preserve cytoskeletal cohesion, and improve deformability. (C) PKR activators increase PKR activity to elevate ATP and lower 2,3-DPG during hypoxia. Higher ATP increases PMCA activity and reduced 2,3-DPG raises Hb-O2 affinity, both of which inhibit Piezo1 and [Ca2+]total accumulation. Restored [Ca2+]total homeostasis attenuates Band 3 phosphorylation and stabilizes the membrane-cytoskeleton complex. (D) GBT021601 combines R-state stabilization with robust 2,3-DPG lowering under hypoxia, suppressing HbSS polymerization and membrane tension. This dual effect limits Piezo1 and supports residual PMCA activity, thereby reducing [Ca2+]total levels, decreasing Band 3 phosphorylation, and markedly enhancing RBC deformability.
Vox, PKR activator, and GBT021601 enhance HbSS RBC deformability under hypoxia through distinct mechanisms. (A) In untreated HbSS cells, increased deoxygenation drives extensive HbSS polymerization, membrane deformation, and stretching; this elevated tension robustly activates Piezo1 channels, leading to marked influx, ATP depletion, and secondary reductions in 2,3-DPG. The resultant [Ca2+]total overload triggers Band 3 phosphorylation, dissociation from the spectrin-actin-protein 4.1 network, and severe loss of deformability. (B) Vox increases Hb-O2 affinity under hypoxia, preventing residual polymer formation and membrane stress. Reduced Piezo1 activation limits influx, whereas improved membrane tension maintains residual PMCA activity. Together, these actions lower intracellular [Ca2+]total, reduce Band 3 phosphorylation, preserve cytoskeletal cohesion, and improve deformability. (C) PKR activators increase PKR activity to elevate ATP and lower 2,3-DPG during hypoxia. Higher ATP increases PMCA activity and reduced 2,3-DPG raises Hb-O2 affinity, both of which inhibit Piezo1 and [Ca2+]total accumulation. Restored [Ca2+]total homeostasis attenuates Band 3 phosphorylation and stabilizes the membrane-cytoskeleton complex. (D) GBT021601 combines R-state stabilization with robust 2,3-DPG lowering under hypoxia, suppressing HbSS polymerization and membrane tension. This dual effect limits Piezo1 and supports residual PMCA activity, thereby reducing [Ca2+]total levels, decreasing Band 3 phosphorylation, and markedly enhancing RBC deformability.
All 3 treatments reduced intracellular [Ca2+]total levels in HbSS RBCs via 2 complementary mechanisms without changing [Ca2+]total levels in HbAA RBCs: (1) inhibiting Piezo1 directly or indirectly and (2) modifying [Ca2+]total efflux pathways, either likely by preserving residual PMCA activity via membrane stabilization or by enhancing PMCA activity via increasing ATP or by activation of alternative Ca2+ clearance pathways. Piezo1 inhibition with GsMTx4 induced further [Ca2+]total reduction, while PMCA inhibition with vanadate increased [Ca2+]total but was reversed by drug effects, confirming that both reduced influx and preserved efflux are essential. Functionally, these corrected [Ca2+]total balance further decreased OI even in the presence of a channel modulator. In interpreting our GsMTx4 results, it is important to recognize that GsMTx4 broadly alters membrane tension and mechanics, affecting multiple mechanosensitive channels beyond Piezo1 alone.51,52 Nevertheless, GsMTx4 has been widely adopted as a functional Piezo1 inhibitor in several RBC studies.25,41 Thus, our observed reductions in [Ca2+]total likely reflect Piezo1 inhibition but may also involve other mechanosensitive pathways. Future studies using more selective Piezo1 inhibitors or direct channel assays are needed to precisely define Piezo1’s role in RBC–calcium homeostasis.
Membrane mechanics play a pivotal role in regulating PMCA function.53 Improving RBC membrane integrity indirectly preserves PMCA activity. Central to the mechano-metabolic link is the Band 3 membrane protein: dephosphorylation of its tyrosine residues enhances ankyrin binding, thereby tightening spectrin-actin linkages and stabilizing the lipid bilayer.8,53,54 A stabilized membrane-cytoskeleton network preserves an ordered lipid microdomain in which PMCA resides, thereby facilitating Ca2+ efflux.53 Band 3 phosphorylation also plays a critical role in RBC homeostasis by modulating membrane-cytoskeleton interactions and cell shape, key determinants of deformability and life span. Phosphorylation of Band 3 disrupts its association with spectrin, actin, and protein 4.1, undermining membrane integrity, impairing ion transporter regulation, and impacting intracellular [Ca2+]total balance and metabolic flux.21,53,55
In this study, voxelotor, GBT021601, and the PKR activator each significantly lowered Band 3 phosphorylation in HbSS RBCs under both normoxic and hypoxic conditions, suggesting improved membrane cytoskeletal stability. Importantly, none of the agents increased ATP levels, a primary substrate for PMCA activity, except PKR activators under hypoxia, suggesting modification of alternative Ca2+ clearance pathways. By preserving PMCA’s lipid-protein microenvironment, membrane stabilization could likely maintain the residual PMCA pump activity despite vanadate inhibition. A more ordered bilayer may unmask secondary Ca2+ efflux pathways such as Na+/Ca2+ exchange or other low-affinity transporters and also alter the buffering through cytosolic Ca2+-binding proteins.56,57 Additionally, studies where ATP was depleted from RBCs have reported a residual PMCA pump activity fueled by ATP generated from the breakdown of the abundant intracellular 2,3-DPG pool.27,58 Since we observed a reduction in 2,3-DPG with GBT021601 and PKR activators under normoxia, we speculate that GBT021601 and PKR activators, by increasing Hb-O2 affinity, promote the catabolism of 2,3-DPG, thereby fueling ATP locally to support residual PMCA efflux activity and contributing to reduced [Ca2+]total and improved deformability.
Elevated [Ca2+]total in HbSS RBCs has been linked to calpain activation,8,47 which cleaves and inactivates protein tyrosine phosphatase 1B (PTP1B), destabilizing interactions among Band 4.1R, Band 3, actin, and spectrin.8,33,55,59 Our selective reduction of Band 3 phosphorylation in HbSS but not HbAA RBCs, under both normoxic and hypoxic conditions, suggests a link between restored [Ca2+]total homeostasis to cytoskeletal stabilization, and thus improved deformability in SCD erythrocytes.
Under normoxia, elevated 2,3-DPG drives low-level HbSS polymer formation, and residual polymers generate membrane tension that activate Piezo1 channels and induce mild Ca2+ influx; this limits the capability to ATP-driven PMCA to Ca2+ efflux and permits calpain activation and Band 3 phosphorylation, thus reducing deformability (Figure 5A); Under hypoxia, accelerated polymerization dramatically heightens membrane stress and Piezo1 activation, driving an increase in [Ca2+]total and, compounded by ATP depletion, further reducing the activity of PMCA-mediated Ca2+ efflux and promoting cytoskeletal destabilization (Figure 6A). Voxelotor counters this cycle by stabilizing HbSS in its oxygenated R-state, reducing residual polymer formation without altering 2,3-DPG or ATP, while inhibiting Piezo1 and modifying Ca2+ clearance pathways (Figures 5C and 6C). GBT021601 shares R-state stabilization but also robustly lowers 2,3-DPG, suppressing polymers, inhibiting Piezo1, and further preserving the residual PMCA activity or by modifying other Ca2+ clearance pathways (Figures 5D and 6D). PKR activators complement these effects by reducing 2,3-DPG, Hb-O2 affinity, inhibiting Piezo1 (Figure 5B), and uniquely elevating ATP under hypoxia (Figure 6B), which increases PMCA activity, thereby preserving membrane-cytoskeleton integrity. Together, these 3 therapeutic interventions converge to prevent calpain activation, limit Band 3 phosphorylation, and re-establish membrane cytoskeleton stability, restoring HbSS RBC deformability under both normoxic and hypoxic stress.
RBC dehydration and deformability are interconnected in SCD pathophysiology, with dehydration further reducing deformability by elevating intracellular HbS concentration, thereby promoting HbS polymerization and increased HbSS RBCs' rigidity.1,7 In our study, treatment with voxelotor, GBT021601, and PKR activators led to significantly decreased OI, suggesting improved RBC deformability underpinned by enhanced membrane stability, as demonstrated by reduced Band 3 phosphorylation. This effect is likely mediated through inhibition of Piezo1 and modulation of Ca2+ clearance pathways, suggesting that the primary therapeutic benefit is restored deformability. However, given the close coupling of cellular volume and shape changes in HbSS RBCs, we cannot fully exclude a concurrent benefit from improved RBC hydration status.
Interestingly, the combination of voxelotor and a PKR activator did not produce additive or synergistic improvements in membrane integrity or Band 3 tyrosine phosphorylation under either normoxic or hypoxic conditions (supplemental Figure 5A-B), suggesting that these agents share overlapping mechanisms that limit the benefit of their combined use. Our findings have underscored the importance of rational combination strategies that employ therapies with mechanistically distinct modes of action, such as hydroxyurea, which induces fetal hemoglobin, or emerging gene-based interventions, to achieve more robust improvements in RBC deformability and clinical outcomes. Although several animal studies and ongoing clinical trials have already demonstrated the individual clinical benefits of these agents, our study reinforces their mechanistic promise in parallel and supports further aggressive therapeutic development.
Importantly, we selected ex vivo concentrations of 600 μM for voxelotor and GBT021601 and 200 μM for the PKR activator based on prior human RBC assays demonstrating meaningful target engagement.15,17,44 Our insights robustly capture changes in [Ca2+]total, 2,3-DPG metabolism, and Band 3 phosphorylation; however, future studies may need to provide detailed dose-response experiments, in vivo models, and direct drug-target interaction assays to confirm these pathways at clinically relevant exposure levels and to guide rational dosing strategies. The clinical relevance of our findings is profound, as they pave the way for regimens that could dramatically reduce SCD morbidity by concurrently correcting key pathophysiological derangements and improving critical markers of anemia, hemolysis, and vaso-occlusive complications. Moreover, these results refine our understanding of the biochemical pathways underlying sickle-RBC deformability and inform the rational design of next-generation combination therapies to further mitigate vaso-occlusive complications and improve patient outcomes in SCD.
Acknowledgments
The authors acknowledge the following funding sources: National Heart, Lung, and Blood Institute (awards R41HL172662 and R44HL140739); National Institute of Biomedical Imaging and Bioengineering (award P41EB021911); National Institute of Allergy and Infectious Diseases (award U01AI176469); and Case-Coulter Translational Research Partnership pilot award. The authors acknowledge institutional support from the Blood Research Center at The University of North Carolina. Z.S. acknowledges the American Society of Hematology Graduate Hematology Award.
This article’s contents are solely the authors' responsibility and do not necessarily represent the official views of the National Institutes of Health.
Authorship
Contribution: Z.S. and U.A.G. conceptualized the idea; Z.S., S.O., B.Z., T.L., F.W., and A.C. assisted with the planning and execution of clinical sample acquisition and testing, including the development of human participant research protocol, patient recruitment, and blood sample collection; Z.S., P.F., A.C., L.W., Y.D., H.B., R.A., J.L., Y.M., and U.A.G. performed the experiments, investigations, data analysis, and visualization; U.A.G. supervised the work, acquired funding, and administrated the project; Z.S. drafted the original manuscript; and all authors reviewed and edited the manuscript.
Conflict-of-interest disclosure: U.A.G. and Case Western Reserve University have financial interests, including licensed intellectual property, stock ownership, research funding, employment, and consulting, in Hemex Health Inc (which offers point-of-care diagnostics for hemoglobin disorders and anemia), BioChip Labs Inc (which offers commercial clinical microfluidic biomarker assays for inherited or acquired blood disorders), and XaTek Inc (which offers point-of-care global assays to evaluate the hemostatic process). U.A.G. has financial interests, including licensed intellectual property, stock ownership, research funding, employment, and consulting in DxNow Inc (which offers microfluidic and bioimaging technologies for in vitro fertilization, forensics, and diagnostics). The competing interests of Case Western Reserve University employees are overseen and managed by the Conflict of Interests Committee according to a Conflict-of-Interest Management Plan. The remaining authors declare no competing financial interests.
Correspondence: Umut A. Gurkan, Case Western Reserve University, 10900 Euclid Ave, Cleveland, OH 44106; email: umut@case.edu.
References
Author notes
All material and data are available upon reasonable request from the corresponding authors, Umut A. Gurkan (umut@case.edu)
The full-text version of this article contains a data supplement.

![Vox, PKR activator, and GBT021601 reduce microcapillary occlusion in HbSS RBCs under normoxia and hypoxia. (A) OcclusionChip device used to assess RBC-mediated microcapillary occlusion under normoxic (pO2 ≈ 100 mm Hg) and mixed venous hypoxic (pO2 ≈ 44 mm Hg) conditions. (B) Microcapillary OI comparison between sickle (HbSS) and healthy (HbAA) RBCs under normoxia (HbAA [n = 8] vs HbSS [n = 15]; ∗∗∗P < .001) and hypoxia (HbAA [n = 8] vs HbSS [n = 15]; ∗∗∗P < .001, Mann-Whitney U test). (C) Under normoxia, treatment with Vox, PKR activator, or GBT021601 significantly reduced OI in HbSS RBCs (HbSS vs HbSS + Vox; HbSS vs HbSS + PKR; HbSS vs HbSS + GBT021601; n = 7; ∗P < .05; Wilcoxon test). (D) Similar reductions were observed under hypoxia (HbSS vs HbSS + Vox, n = 12 [∗∗∗P < .001]; HbSS vs HbSS + PKR, n = 12 [∗∗∗P < .001]; HbSS vs HbSS + GBT021601, n = 6 [∗∗∗P < .001]; Wilcoxon test). (E) Summary of mean ± SEM OI for HbAA, HbSS, and treated HbSS RBCs (HbSS + Vox, HbSS + PKR, and HbSS + GBT021601) under normoxic and hypoxic conditions. Error bars are presented as SEM. ns, not significant; SEM, standard error of the mean; Vox, voxelotor.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodrci/1/2/10.1016_j.brci.2025.100015/3/m_brci_rci-2025-000108-gr1.jpeg?Expires=1769197758&Signature=jxja0TircwjbFqZUnq3DsZe2bVGdrFDdSJ3rvDIIUNO9W07tW1wfp7tBqqWec-A~~KlmMReRB0QLQSAgIw0uGrUzvn1HraXkXxB97~iVj04Q3Ku9AWyIT4398QGfYKY3I4vvc2SKwiMnMgiCQcAo52UfaJfR~4cPZ-NXTZrBzESyfhqKtHkYAzcygx3FHgF18AhyFzSICsYERRgIoAdP~iy6lNSPtmAU1t0PdPHOILK5UhifQjkwC4OEUm8QY2Hwhq6-r2JlWg8vmyg-2L-e9G0fofigIvEy-2dNuyaCxqFnxjyrFq5lTRCNoadY~Q9A6L4~K7AozSVLJ49cffMy7w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![PKR activator or GBT021601 reduces 2,3-DPG levels in HbSS RBCs and modulates ATP under normoxia and hypoxia. (A) Under normoxia, HbAA RBCs exhibited higher ATP levels compared to HbSS RBCs (HbAA [n = 5] vs HbSS [n = 27]; ∗P < .05; Mann-Whitney U test). ATP levels in untreated HbSS RBCs were unaffected by treatment with Vox, PKR activator, or GBT021601 (ns, P > .05; Wilcoxon test). (B) Normoxic 2,3-DPG levels were lower in HbAA RBCs than in HbSS RBCs (HbAA, n = 5 vs HbSS, n = 27; ∗P < .05, Mann-Whitney U test). PKR activator and GBT021601 reduced 2,3-DPG levels in HbSS RBCs (HbSS vs HbSS + PKR [n = 27; ∗∗P < .01; Wilcoxon test]; HbSS vs HbSS + GBT021601 [n = 5; ∗∗P < .01; Wilcoxon test]), whereas Vox had no effect (ns, P > .05; Wilcoxon test). (C) Under hypoxia, HbAA RBCs retained higher ATP levels than HbSS RBCs (HbAA [n = 5] vs HbSS [n = 10]; ∗P < .05; Mann-Whitney U test). PKR treatment increased ATP levels (HbSS vs HbSS + PKR; n = 10; ∗∗P < .01; Wilcoxon test), whereas Vox and GBT021601 had no effect (ns; P > .05; Wilcoxon test). (D) Similar to normoxia, hypoxic 2,3-DPG levels were lower in HbAA RBCs compared to HbSS RBCs (HbAA [n = 5] vs HbSS [n = 10]; ∗P < .05; Mann-Whitney U test). PKR activator and GBT021601 reduced 2,3-DPG levels (HbSS vs HbSS + PKR; ∗P < .05; HbSS vs HbSS + GBT021601; ∗P < .05; Wilcoxon test), whereas Vox had no effect (ns, P > .05; Wilcoxon test). Error bars are presented as SEM. ns, not significant.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodrci/1/2/10.1016_j.brci.2025.100015/3/m_brci_rci-2025-000108-gr2.jpeg?Expires=1769197758&Signature=se1lr1b~JoQ53EsyFePOom3a9T~O72WvkT4VA9IvD5XZdPQZcl8KNeqvfnxrg8J37xt1T0LFwlNzT60E7WN43ipE6qtNvNQjLVJwaqKmQ9eHW4LBeaQWT8hvo1SEufLCEz3k2yhbXPO3CfV6VqKCSxlejnb7lSDZv95P54jIoWRo3tzo93lTLIUYtVqRmYdYFO28Kd17W~ngMdP3wPxN9SSqLWNOonlxixBCwVemAwNP9jPZRKRvbHdTcuT1bB-8VM~~dR71jsYVgujyj979qVcRfZLnHqGpR1l6H-YDal8haiqy0ViuG8Yrf2vD9OFGrL0R9Ykm4hKPEOL26fSpOA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Vox, PKR activator, and GBT021601 reduced [Ca2+]total levels and improved HbSS RBC occlusion by Piezo1 inhibition and enhancing residual PMCA activity under normoxic and hypoxic conditions. (A) In HbAA RBCs, Vox and PKR activator did not change the basal intracellular [Ca2+]total levels (n = 5; ns, P > .05; Wilcoxon test). (B) Under normoxia, [Ca2+]total levels were significantly reduced in HbSS RBCs treated with Vox (HbSS vs HbSS + Vox; n = 21), PKR activator (HbSS vs HbSS + PKR; n = 21), or GBT021601 (HbSS vs HbSS + GBT021601; n = 5), ∗∗∗P < .001, t test for all. Relatively greater reductions were observed under hypoxia (HbSS + Vox, n = 6; HbSS + PKR, n = 6; HbSS + GBT021601, n = 6; ∗∗∗P < .001; Wilcoxon test for all). (C) Piezo1 blockade with GsMTx4 reduced intracellular [Ca2+]total levels under normoxia and hypoxia, but did not differ significantly with cotreatment with Vox, PKR activator, and GBT021601 (∗∗∗P < .001; n = 5; Wilcoxon test for all). (D) PMCA inhibition with vanadate increases [Ca2+]total levels under both normoxia and hypoxia; cotreatment with Vox, PKR activator, and GBT021601 significantly lowered the [Ca2+]total levels despite PMCA blockade (∗∗∗P < .001; n = 5; Wilcoxon test for all). (E) Similar to [Ca2+]total levels, OI under GsMTx4 alone was reduced compared with untreated HbSS RBCs (∗P > .05; n = 5; Wilcoxon test), and did not differ significantly with cotreatment with Vox, PKR activator, and GBT021601 (ns, P > .05; n = 7; t test for all). (F) Vanadate increased OI under both normoxia (n = 5; ∗P < .05; Wilcoxon test) and hypoxia (n = 5; ∗∗∗P < .001; Wilcoxon test). Cotreatment with Vox, PKR activator, or GBT021601 significantly lowered the OI (n = 5; ∗P < .05, ∗∗P < .01, ∗P < .05; Wilcoxon test). Error bars are presented as mean ± SEM. Gs, Grammostola spatulata mechanotoxin 4.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodrci/1/2/10.1016_j.brci.2025.100015/3/m_brci_rci-2025-000108-gr3.jpeg?Expires=1769197758&Signature=pDHjl-AA1nV3lVK7C8Vq~oE9z93Ya~XVj6WKEPWWn8f92GZoYVzChtXHpXzh78U7ZfFaICM6cJHyVijChzN3wQ0VYRIHhbZBG~w9uTYh3AakInMrhWtTnX6r7ol-ob-1VUjULrJK3oWO93PJ-KwZueIlAESGS-PZKc1V1lynZY9hMhbFgNyR1vZewdXtJEvzNJKJFPPS7guNQh7bypeu5Yo3SORH1s8BUKDoFq5hO46DKAJTiBcIUcrNHg7agbHbd6~~KQDL9TQRqa47MotMAzCUatoQ6NAoc7MjhAten5tq0vW60CViu673f83qYrENEnle~FeYK6D2w0nUnt7gvQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Vox, PKR activator, and GBT021601 reduce Band 3 tyrosine phosphorylation in HbSS RBCs under normoxia and hypoxia. (A) A total of 20 μg of total protein was loaded per lane for all immunoblots. Representative anti–Band 3 immunoblots for untreated and treated HbAA and HbSS RBCs (Vox, PKR activator, GBT021601). (B) Band 3 tyrosine phosphorylation levels in HbAA RBCs were unaffected by treatment with Vox or PKR activator (HbAA vs HbAA + Vox and HbAA + PKR; n = 7; ns, P > .05; Wilcoxon test). (C) Under normoxia, Band 3 tyrosine phosphorylation levels were significantly reduced in HbSS RBCs treated with Vox (HbSS vs HbSS + Vox; n = 22; ∗∗∗P < .001; t test), PKR activator (HbSS vs HbSS + PKR; n = 22; ∗∗∗P < .001; t test), and GBT021601 (HbSS vs HbSS + GBT021601; n = 5; ∗P < .05; t test). (D) Similar reductions were observed under hypoxia (HbSS + Vox [n = 10; ∗∗P < .01]; HbSS + PKR [n = 10; ∗∗P < .01]; HbSS + GBT021601 [n = 10; ∗∗P < .01; t test]). Error bars are presented as mean ± SEM.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodrci/1/2/10.1016_j.brci.2025.100015/3/m_brci_rci-2025-000108-gr4.jpeg?Expires=1769197758&Signature=WcvvhtqILXchjdbRT4n7r-sijwmdnNybkrJX-Tv-vJkM~wNXkdgYzDFSn48bjgQMjk64biNEcIeUJWRX5G~zlJrOuozCk~LcmgMfckew4JG-wan-p~gCTvsQM1I-H6fyUd80HHKeSTKf1-Act2WCHTs7PMRVjDtabX2pGgS58XsjNESDHf3UJnPFjZ9NJGGG871S-hZ~dItvYnqJO1gDOg8Mjo4awZWsdXwnHeW9cnvr22E12cxUuWFAW4jITubFzw2Zfbb2BWWTTfBNrz3SZ7-o1f4wGZ0~2n3QUdq~IbejZd0CqljR3CK3ODap4rqD8My3rWFxm0OrjlpjOQJ-Jw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Vox, PKR activator, and GBT021601 improve HbSS RBC deformability via distinct pathways under normoxia. (A) In untreated HbSS cells, residual deoxy-HbS polymers are present, even at relatively high levels of oxygenation, and they are exacerbated by elevated 2,3-DPG, which imposes membrane tension that activates Piezo1 and elicits influx. Concurrent ATP depletion impairs PMCA, leading to [Ca2+]total overload, calpain activation, and Band 3 phosphorylation. Phosphorylated Band 3 dissociates from the spectrin-actin-protein 4.1 network, disrupting membrane cytoskeleton cohesion and thereby reducing deformability. (B) Vox covalently stabilizes HbSS in its R-state, reducing deoxy-HbS polymer formation without altering ATP or 2,3-DPG. Lower membrane tension limits Piezo1 opening and influx, normalizing [Ca2+]total. Reduced [Ca2+]total results in Band 3 dephosphorylation, maintains PMCA microdomains, and preserves cytoskeletal integrity, thereby improving deformability. (C) PKR activators enhance PKR activity to lower 2,3-DPG. Reduced 2,3-DPG increases Hb-O2 affinity, suppresses polymerization, and decreases membrane stress–induced Piezo1 activation. By preserving residual PMCA function and restoring Ca2+ homeostasis, PKR activators reduce Band 3 tyrosine phosphorylation and stabilize the spectrin-actin-protein 4.1 cytoskeletal complex, markedly improving RBC membrane integrity and deformability. (D) GBT021601 combines R-state stabilization with robust 2,3-DPG lowering. The dual effect suppresses HbS polymerization, reduces membrane tension, inhibits Piezo1, and maintains residual PMCA activity. The resulting [Ca2+]total reduction limits Band 3 phosphorylation, stabilizes the membrane-cytoskeleton network, and markedly enhances RBC deformability.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodrci/1/2/10.1016_j.brci.2025.100015/3/m_brci_rci-2025-000108-gr5.jpeg?Expires=1769197758&Signature=dLN-7OqIy-omSbIjdKI87LUfPB~sD81eed1fgMYdB1~nDDbUYfb1FGidt0GWWR5MDhEkA1LZSO14ITGKOrq1GvnvPeHgq-cAyeZwoC~UJLnQJu4chxLzZEFmvhbIvOPjJO8WfyUPgeTZnh9l3zr9DnBx5gLkjldjiy7jhzbrYqvXgOrOHnou~9OGjiMsYGRmBxIPlU1h~2UQ2HSjhoU7L6xry1w8dUqE10zO4vEGTrU5ut9sg6hKGCfXIi2ObSzD1DCLbVkW0jeKZPeP0WuzReloStV~ivv-M0RT1vYIcxWW1k-aXbCqmdsPr6FRMltTYCuo2g4VR0jKngURro14hA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Vox, PKR activator, and GBT021601 enhance HbSS RBC deformability under hypoxia through distinct mechanisms. (A) In untreated HbSS cells, increased deoxygenation drives extensive HbSS polymerization, membrane deformation, and stretching; this elevated tension robustly activates Piezo1 channels, leading to marked influx, ATP depletion, and secondary reductions in 2,3-DPG. The resultant [Ca2+]total overload triggers Band 3 phosphorylation, dissociation from the spectrin-actin-protein 4.1 network, and severe loss of deformability. (B) Vox increases Hb-O2 affinity under hypoxia, preventing residual polymer formation and membrane stress. Reduced Piezo1 activation limits influx, whereas improved membrane tension maintains residual PMCA activity. Together, these actions lower intracellular [Ca2+]total, reduce Band 3 phosphorylation, preserve cytoskeletal cohesion, and improve deformability. (C) PKR activators increase PKR activity to elevate ATP and lower 2,3-DPG during hypoxia. Higher ATP increases PMCA activity and reduced 2,3-DPG raises Hb-O2 affinity, both of which inhibit Piezo1 and [Ca2+]total accumulation. Restored [Ca2+]total homeostasis attenuates Band 3 phosphorylation and stabilizes the membrane-cytoskeleton complex. (D) GBT021601 combines R-state stabilization with robust 2,3-DPG lowering under hypoxia, suppressing HbSS polymerization and membrane tension. This dual effect limits Piezo1 and supports residual PMCA activity, thereby reducing [Ca2+]total levels, decreasing Band 3 phosphorylation, and markedly enhancing RBC deformability.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodrci/1/2/10.1016_j.brci.2025.100015/3/m_brci_rci-2025-000108-gr6.jpeg?Expires=1769197758&Signature=Sbqt3LZQBd-OQzEfLgqMs7IYU6TfP9tU3DhIWGMttnJJz9TMRaeaqzy~U8KW8re9wtxrb3isQVtKiJhYTvy-OL7KcrH5y5bFZs8Y6T9OtiQ-wtczbJxm6kl7WHQJ23z68WfMLAZUFOdsVHvaixyH~gf8RO7rw5jEQrEvKusUQEyZSSGJKVyppNkBcJf8waXoLWiFOi~RCHaMQBaW5rVwdMAbFjpGX9Ij1r-DOpNyGzmOQHy9PCB5r4IKEdKeKCh5YsUBtTnIOrTzzePChRo0zYazgGBNeXuJeDBdV-91EaUHTmVa9DplTvx7-HG1XfHqT42zomA1nacrrZd01mWFWQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)