Key Points
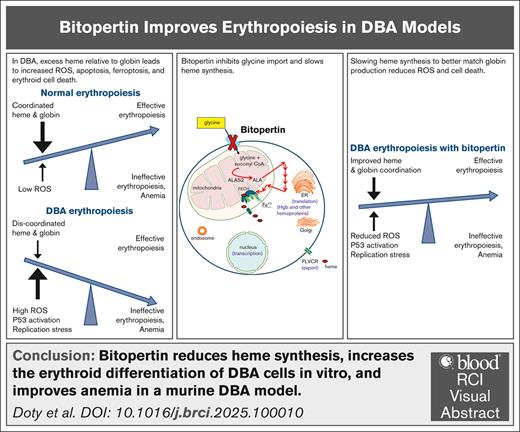
Bitopertin reduces heme synthesis, increases the erythroid differentiation of DBA cells in vitro, and improves anemia in a murine DBA model.
The data justify a trial of bitopertin in patients with DBA and suggest a lower dose than that used in unrelated trials could be efficacious.
Visual Abstract
Diamond-Blackfan anemia (DBA) results from germ line haploinsufficiency of 1 of at least 26 distinct ribosomal proteins. Although patients with DBA have hematopoietic stem and progenitor cell defects, the dominant clinical phenotype is severe anemia. In ∼60% of patients with DBA, the anemia responds to corticosteroids. However, these responses are often time limited, and steroid-related complications are common, leaving an unmet need for an effective and safe oral therapy. In DBA, ribosomal haploinsufficiency leads to slowed translation and impaired protein synthesis. Globin synthesis is significantly slowed, whereas the production of heme, which requires a small amount of protein because it is synthesized enzymatically, proceeds at a near normal rate. This results in an excess of intracellular heme in early erythroblasts, elevated reactive oxygen species, and other heme-induced toxicity. Bitopertin, an oral competitive inhibitor of glycine import, has been shown to reduce heme synthesis and to have an excellent safety profile in unrelated phase 2 and 3 studies. We reasoned that bitopertin might help balance heme synthesis with globin synthesis and improve erythropoiesis in patients with DBA. Our observations in samples from patients with DBA, CD34+ cells engineered to downregulate RPS19, and a murine DBA model support this concept, justify ongoing clinical studies, and provide insight into optimal trial design.
Introduction
Diamond-Blackfan anemia (DBA) is a rare congenital marrow failure syndrome. It presents as a hypoplastic anemia. Although skeletal anomalies, short stature, mild other cytopenias, and an increased cancer incidence are seen, severe anemia is the most pervasive morbidity.1 The anemia does not respond to erythropoietin (baseline erythropoietin levels are high in patients with DBA), but ∼60% of patients respond to corticosteroids. However, this response is often transient, and steroid toxicities and resistance are common. Only 30% to 40% of individuals remain on steroid therapy as chronic management.1 Red cell transfusions result in iron overload and thus require ongoing chelation. Hematopoietic stem cell transplant has limited availability and carries the risk of graft-versus-host disease and other complications, plus it is uncertain if myeloablative preparative regimens will increase the incidence of solid tumors. Drugs such as leucine and metoclopramide have had rare successes.1 New, safe, and preferably simpler therapies are needed.
DBA results from the heterozygous deletion, missense, splice or frameshift mutation, and haploinsufficiency of any 1 of at least 26 different ribosomal proteins.1-3 In addition, it has been associated with mutations of TRS2 and HEATR3, which are involved in ribosome assembly. That so many distinct genetic abnormalities result in ineffective erythropoiesis and profound anemia suggests that ribosome dysfunction, impaired translation, and slowed protein synthesis underlie its pathogenesis. When protein synthesis is slowed, globin synthesis is slowed, whereas the synthesis rate of heme (a toxic chelate constructed enzymatically) proceeds at a near normal rate. This results in excess heme relative to globin in colony-forming unit erythrocyte and proerythroblasts; increased reactive oxygen species, oxidative stress, ferroptosis, and apoptosis.4 Oxidative stress also leads to p53 activation, which is another common finding in DBA.3,5,6 A third finding characterizing DBA is inappropriately low levels of the erythroid-megakaryocytic transcription factor GATA-binding protein 1 (GATA1), the master regulator that initiates and maintains erythropoiesis.7,8 Notably, heme transcriptionally downregulates GATA1 and also binds heat shock protein 70, inhibiting its ability to stabilize GATA1 protein.9,10 Thus, excessive heme not only directly damages early erythroblasts, but also could decrease GATA1, leading to the premature termination of the erythroid differentiation program.
We reasoned that balancing heme synthesis with the slowed globin synthesis might offset heme toxicities, allowing erythropoiesis to proceed more robustly. Consistent with this concept, slowing heme synthesis with succinylacetone, a specific inhibitor of the second step of the heme synthetic pathway, or via iron restriction improved red cell production from progenitor cells from patients with DBA in vitro and in a Flvcr1-deleted DBA-like murine model in vivo.4,11 However, succinylacetone is the toxic metabolite in hereditary tyrosinemia, and iron restriction also has broader consequences and so these are not viable or optimal therapies for patients with DBA.
In the first step of heme biosynthesis, glycine and succinyl-CoA combine to form aminolevulinic acid. Although glycine is a nonessential amino acid, erythroid precursors are unable to synthesize sufficient glycine to support robust heme synthesis and rely on glycine import via the glycine transporter 1 (SLC6A9).12 Bitopertin is an oral small-molecule inhibitor of glycine transporter 1.13 In phase 2 and 3 clinical studies of schizophrenia, it was found to be ineffective, but exhibited a favorable safety profile.14,15 More recently, it was shown to improve anemia in a mouse model of β-thalassemia16 and reduce the accumulation of phototoxic intermediates in a murine model of erythropoietic protoporphyria (EPP),17 a disorder caused by mutations leading to reduced function of ferrochelatase, the enzyme catalyzing the final step in the heme synthetic pathway. Bitopertin was similarly effective in reducing phototoxicity in a phase 2 study enrolling 75 EPP patients,18 prompting a phase 3 study with patients aged as young as 12 years, which is currently underway.
Here we confirm that bitopertin reduces erythroid heme synthesis. We demonstrate that it improves the in vitro erythroid output from marrow from patients with DBA and CD34+ hematopoietic stem and progenitor cells engineered to downregulate ribosomal protein S19 (RPS19), but not normal marrow or hematopoietic stem and progenitor cells. In addition, bitopertin treatment results in a dose-dependent improvement in anemia in the Rpl11-haploinsufficient murine DBA model.
Study design
Human marrow and cord blood cultures
Marrow samples were collected and cryopreserved after written informed consent on Institutional Review Board-approved protocols according to the Declaration of Helsinki. CD34+ cells and CD14+ monocytes were isolated from cord blood samples (Bloodworks Northwest). Cells were cultured in a multiphase erythroid differentiation culture system19 with a modified timeline. Thawed marrow cells were placed in expansion media overnight. After Ficoll separation, viable cells were cultured at 2 × 105 cells per mL in differentiation media I, II, and III for days 1 to 10, 10 to 13, and 13 to 21, respectively, correcting concentrations to 2 × 105 cells per mL every 3 to 4 days. Erythroid differentiation was characterized as before.4 CD34+ cells were transduced with either control (shLuc)20 or shRPS1921 in the pLKO.1 vector22 expressing blue fluorescent protein. Successfully transduced CD34+ cells were isolated by sorting and then cultured in differentiation media I. CD14+ monocytes were matured to macrophages as described.23 As 10 nM bitopertin maximized the erythroid cell output of cultures with marrow from patients with DBA, this concentration was used in all cultures.
Animal studies
Adult male and female Rpl11-haploinsufficient mice were generated as before.24 After anemia was confirmed via complete blood count, the mice were switched to iron-sufficient diets (50 ppm) containing 0 to 150 ppm bitopertin (Inotiv-Teklad diets, Madison, WI). Blood and marrow isolation and flow cytometric analysis were performed as before.11 Plasma bitopertin levels were determined by liquid chromatography-mass spectrometry/mass spectrometry (Resolian Inc, Malvern, PA). All animal studies were approved by the University of Washington Institutional Animal Care and Use Committee.
Statistical analysis
Statistical analyses were performed with Excel or GraphPad Prism. Data are shown as the mean ± standard error of the mean or mean ± standard deviation as indicated. P ≤ .05 was considered statistically significant.
Results and discussion
Because RPS19 haploinsufficiency is the most common mutation (∼25%) in patients with DBA,1 we used human cord blood CD34+ cells transduced with shRPS19 as a model. CD34+ cells transduced with shRPS19 expressed 44 ± 2% less RPS19 messenger RNA than cells transduced with a luciferase control short hairpin RNA and expanded poorly in culture. Bitopertin improved the erythroid expansion of the RPS19-deficient precursors 63 ± 13% (P < .001), whereas the erythroid expansion of control (shLuc) precursors was not significantly impacted (Figure 1A). As red cell differentiation takes place in the context of an erythroblastic island (EBI) niche, we next cultured the shRPS19 and control erythroblasts with cord blood–derived macrophages in EBI cultures using published methods.23 Bitopertin increased the expansion of RPS19-deficient CD34+ cell–derived erythroblasts 3.2 ± 0.3-fold (P < .0001) during the 3-day EBI culture, whereas it inhibited the expansion of control erythroblasts (Figure 1A).
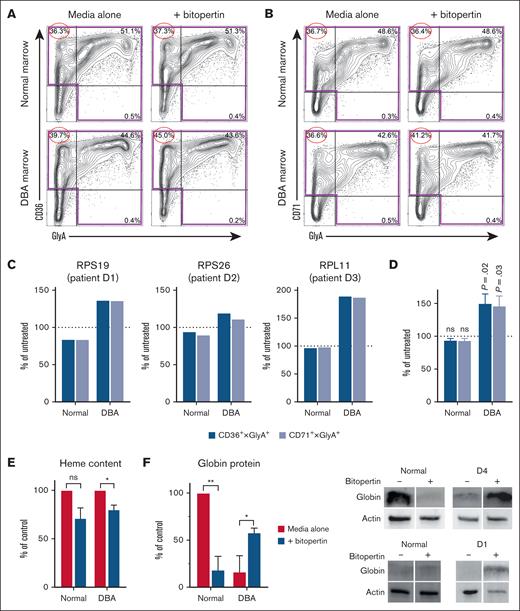
Bitopertin increases the erythroid expansion of marrow from patients with DBA in culture. (A) Blue fluorescent protein positive sorted CD34+ cells expressing either control (shLuc) or RPS19 short hairpin RNA (shRNA) were cultured for 10 days in differentiation media I without or with 10 nM bitopertin (left). The erythroid precursors were then cultured in an EBI culture with CD14-derived macrophage for an additional 3 days in differentiation media II without or with bitopertin (right). Data are presented as the mean and standard error of the mean of the numbers of cells recovered from 3 independent experiments (control: n = 3, RPS19 shRNA: n = 6 [3 each of 2 independent shRNAs]) after normalizing cell counts to reflect starting each culture with 200 000 cells. (B) Characteristics of marrow samples from patients with DBA and control subjects used in studies. (C) Representative flow cytometry analyses of cryopreserved marrow mononuclear cells from a patient with DBA (D2) and a control subject (N1) before and after 3 days in erythroid culture show that erythroid differentiation in patients with DBA fails in vivo at the colony-forming unit erythrocyte and/or proerythroblast stage when CD71 is upregulated, but in vitro culture allows continued differentiation. (D) A representative time course showing the total cell expansion and CD71+ cell expansion of marrow cells from normal (N1) and a patient with DBA (D1) cultured without or with bitopertin. (E) Total cell expansion or expansion of CD71+ cells in marrow cultures from control subjects and patients with DBA for 10 days in differentiation media I without or with bitopertin. Cell counts from each sample were normalized to the culture without bitopertin and presented as a percent of control (n = 4). ∗P < .05; ∗∗∗P < .001; ∗∗∗∗P < .0001; ns, P > .05. ns, not significant.
Bitopertin increases the erythroid expansion of marrow from patients with DBA in culture. (A) Blue fluorescent protein positive sorted CD34+ cells expressing either control (shLuc) or RPS19 short hairpin RNA (shRNA) were cultured for 10 days in differentiation media I without or with 10 nM bitopertin (left). The erythroid precursors were then cultured in an EBI culture with CD14-derived macrophage for an additional 3 days in differentiation media II without or with bitopertin (right). Data are presented as the mean and standard error of the mean of the numbers of cells recovered from 3 independent experiments (control: n = 3, RPS19 shRNA: n = 6 [3 each of 2 independent shRNAs]) after normalizing cell counts to reflect starting each culture with 200 000 cells. (B) Characteristics of marrow samples from patients with DBA and control subjects used in studies. (C) Representative flow cytometry analyses of cryopreserved marrow mononuclear cells from a patient with DBA (D2) and a control subject (N1) before and after 3 days in erythroid culture show that erythroid differentiation in patients with DBA fails in vivo at the colony-forming unit erythrocyte and/or proerythroblast stage when CD71 is upregulated, but in vitro culture allows continued differentiation. (D) A representative time course showing the total cell expansion and CD71+ cell expansion of marrow cells from normal (N1) and a patient with DBA (D1) cultured without or with bitopertin. (E) Total cell expansion or expansion of CD71+ cells in marrow cultures from control subjects and patients with DBA for 10 days in differentiation media I without or with bitopertin. Cell counts from each sample were normalized to the culture without bitopertin and presented as a percent of control (n = 4). ∗P < .05; ∗∗∗P < .001; ∗∗∗∗P < .0001; ns, P > .05. ns, not significant.
We used marrow cultures from patients with DBA (Figure 1B) to validate these findings. Erythroid differentiation in vivo fails early, often when CD71 is first upregulated. However, in culture, DBA erythroid cells can continue their differentiation, which allows for in vitro assessment (Figure 1C). As shown in Figure 1D, when marrow from patients with DBA was cultured with bitopertin, the total number of cells and the number of CD71+ erythroid precursors increased. Bitopertin treatment resulted in an average 8% increase in total cells and a 22% increase in the number of CD71+ erythroid precursors after 10 days (Figure 1E) despite substantial variability in the extent and kinetics of expansion (eg, Figure 1D).
Consistent with our concept that heme-globin imbalance is maximal during early erythropoiesis, flow cytometric studies showed that bitopertin specifically increased the numbers of early-midstage (CD36+ or CD71+) erythroid cells (Figure 2A-C). Combined peak expansion data for the DBA cultures showed bitopertin significantly increased the numbers of CD36+ and CD71+ erythroid precursors (Figure 2D) relative to untreated cultures. By day 7, the heme content decreased in cells from both control subjects and patients with DBA (Figure 2E), confirming that inhibiting glycine import impairs heme synthesis. Despite substantial variability in the amount of globin present in marrow cultures from normal donors, bitopertin treatment reduced the globin content of control cells at culture day 7 (Figure 2F). This is consistent with the expected regulation of globin translation via the heme-regulated inhibitor kinase eIF2a. In contrast, bitopertin increased globin protein levels 3.5-fold in the marrow cultures from a patient with DBA (Figure 2F). This reflects the improved erythroid maturation observed in Figure 2D.
Bitopertin improves the erythroid cell maturation of marrow from patients with DBA. (A-B) Representative flow cytometry analysis of marrow mononuclear cells from a patient with DBA (D1) and a control subject (N1), cultured without or with 10 nM bitopertin after 10 days of culture. Lin– (lineage negative: CD3−, CD11b−, CD19−) cells from each culture were plotted as CD36 × GlyA (A) or CD71 × GlyA (B), showing the percentage of lin− cells in each quadrant. The largest improvement in the DBA marrow cultures is seen in the earliest precursors (CD36 or CD71 single positive), which are circled. (C-D) A total of 200 000 marrow mononuclear cells from control subjects or patients with DBA with mutations in RPS19, RPS26, or RPL11 were cultured for 7 to 13 days without or with bitopertin. The total number of erythroid cells in each culture was calculated from the number of cells in the highlighted quadrants using either CD36 × GlyA or CD71 × GlyA (see panels A-B). The day shown for each DBA sample is the day that each DBA sample showed peak improvement with bitopertin treatment, which was different for each patient’s marrow culture. Data are presented as the percentage of untreated which was calculated by dividing the numbers of erythroid cells recovered from the bitopertin treated cultures by the number of cells recovered from the untreated cultures individually for each control and patient culture (C) or as the mean ± standard error of the mean of all 4 patient and control cultures (D) at the day that each patient culture showed peak improvement. P values are shown for the comparison of the treated vs untreated (100%). (E) Relative heme content of bone marrow cultures from control subjects or patients with DBA after 7 days of culture without or with bitopertin. Samples were collected and analyzed for total heme content as before.4,11 Data are presented as the mean ± standard error of the mean (of 3 independent experiments) of the treated samples relative to the heme content of the untreated samples. (F) Relative amounts of globin protein in marrow cultures from control subjects or patients with DBA after 7 days of culture without or with bitopertin (left). Total protein was isolated from samples and analyzed via western blot (right) as before.4 Fluorescent images of the blots were captured on a Bio-Rad ChemiDoc MP and processed in Image Lab. Data are presented as a percentage of globin present in the untreated control sample after correcting for loading using actin (n = 2 independent experiments). ∗P < .05; ∗∗P < .01; ns, P > .05. ns, not significant.
Bitopertin improves the erythroid cell maturation of marrow from patients with DBA. (A-B) Representative flow cytometry analysis of marrow mononuclear cells from a patient with DBA (D1) and a control subject (N1), cultured without or with 10 nM bitopertin after 10 days of culture. Lin– (lineage negative: CD3−, CD11b−, CD19−) cells from each culture were plotted as CD36 × GlyA (A) or CD71 × GlyA (B), showing the percentage of lin− cells in each quadrant. The largest improvement in the DBA marrow cultures is seen in the earliest precursors (CD36 or CD71 single positive), which are circled. (C-D) A total of 200 000 marrow mononuclear cells from control subjects or patients with DBA with mutations in RPS19, RPS26, or RPL11 were cultured for 7 to 13 days without or with bitopertin. The total number of erythroid cells in each culture was calculated from the number of cells in the highlighted quadrants using either CD36 × GlyA or CD71 × GlyA (see panels A-B). The day shown for each DBA sample is the day that each DBA sample showed peak improvement with bitopertin treatment, which was different for each patient’s marrow culture. Data are presented as the percentage of untreated which was calculated by dividing the numbers of erythroid cells recovered from the bitopertin treated cultures by the number of cells recovered from the untreated cultures individually for each control and patient culture (C) or as the mean ± standard error of the mean of all 4 patient and control cultures (D) at the day that each patient culture showed peak improvement. P values are shown for the comparison of the treated vs untreated (100%). (E) Relative heme content of bone marrow cultures from control subjects or patients with DBA after 7 days of culture without or with bitopertin. Samples were collected and analyzed for total heme content as before.4,11 Data are presented as the mean ± standard error of the mean (of 3 independent experiments) of the treated samples relative to the heme content of the untreated samples. (F) Relative amounts of globin protein in marrow cultures from control subjects or patients with DBA after 7 days of culture without or with bitopertin (left). Total protein was isolated from samples and analyzed via western blot (right) as before.4 Fluorescent images of the blots were captured on a Bio-Rad ChemiDoc MP and processed in Image Lab. Data are presented as a percentage of globin present in the untreated control sample after correcting for loading using actin (n = 2 independent experiments). ∗P < .05; ∗∗P < .01; ns, P > .05. ns, not significant.
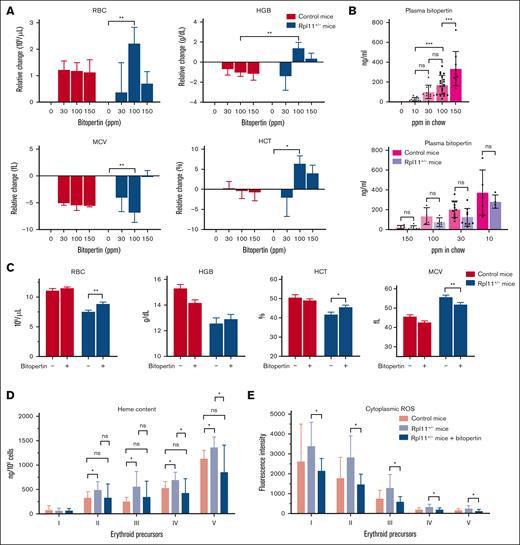
To determine if bitopertin could improve anemia in vivo, we treated Rpl11-haploinsufficient mice, a DBA model. These mice have ineffective erythropoiesis and anemia, as well as excess erythroblast heme.24,25 We tested a range of bitopertin concentrations provided ad libitum in chow. There was a dose-dependent improvement in anemia with 100 ppm bitopertin (20 mg/kg per day) being the optimal dose (Figure 3A). This resulted in an average plasma concentration of 169 ng/mL in plasma samples collected 3 to 5 hours after lights were on (Figure 3B). The improvement in the red blood cells and hemoglobin levels was less pronounced with the 150 ppm bitopertin than with the 100 ppm bitopertin. Interestingly, 200 ppm bitopertin was required to optimize responses in mouse models of EPP17 and β-thalassemia.16
Bitopertin improves erythropoiesis in the Rpl11-haploinsufficient mouse model of DBA. (A) Mice were fed chow containing 0, 30, 100, or 150 ppm for 8 weeks to identify the optimal dose of bitopertin. Changes in red cell complete blood count parameters relative to untreated mice are shown as mean ± standard deviation (SD) of 4 to 6 mice. Mice treated with 100 ppm bitopertin in chow (∼20 mg/kg per day) showed the best improvement. (B) Bitopertin levels in plasma were measured and shown for each treatment dose (top) or segregated by control and haploinsufficient mice (bottom). Plasma samples were collected 3 to 5 hours after light-on and generally reflect the nadir. Data from each mouse is shown individually along with the mean ± SD for each group. (C) Red cell complete blood count parameters of control and Rpl11-haploinsufficient mice treated and fed a controlled diet without and with 100 ppm bitopertin for 8 weeks. Data are presented as mean ± SD of 11 to 15 mice. Heme content (D) and cytoplasmic reactive oxygen species (E) in marrow erythroid precursors from control mice or Rpl11-haploinsufficient mice treated without or with 100 ppm bitopertin. Analysis was performed as before11 and presented as the mean heme content ± SD (7-8 mice) or the mean fluorescence intensity of CM-DCFDA ± SD (4-6 mice) of at each stage of differentiation (I, colony-forming unit erythrocyte and proerythroblasts; II, basophilic erythroblasts; III, polychromatic erythroblasts; IV, orthochromatic erythroblasts and reticulocytes; V, retics and RBC). ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ns, P > .05. HCT, hematocrit; HGB, hemoglobin; MCV, mean corpuscular volume; ns, not significant; RBC, red blood cell; ROS, reactive oxygen species.
Bitopertin improves erythropoiesis in the Rpl11-haploinsufficient mouse model of DBA. (A) Mice were fed chow containing 0, 30, 100, or 150 ppm for 8 weeks to identify the optimal dose of bitopertin. Changes in red cell complete blood count parameters relative to untreated mice are shown as mean ± standard deviation (SD) of 4 to 6 mice. Mice treated with 100 ppm bitopertin in chow (∼20 mg/kg per day) showed the best improvement. (B) Bitopertin levels in plasma were measured and shown for each treatment dose (top) or segregated by control and haploinsufficient mice (bottom). Plasma samples were collected 3 to 5 hours after light-on and generally reflect the nadir. Data from each mouse is shown individually along with the mean ± SD for each group. (C) Red cell complete blood count parameters of control and Rpl11-haploinsufficient mice treated and fed a controlled diet without and with 100 ppm bitopertin for 8 weeks. Data are presented as mean ± SD of 11 to 15 mice. Heme content (D) and cytoplasmic reactive oxygen species (E) in marrow erythroid precursors from control mice or Rpl11-haploinsufficient mice treated without or with 100 ppm bitopertin. Analysis was performed as before11 and presented as the mean heme content ± SD (7-8 mice) or the mean fluorescence intensity of CM-DCFDA ± SD (4-6 mice) of at each stage of differentiation (I, colony-forming unit erythrocyte and proerythroblasts; II, basophilic erythroblasts; III, polychromatic erythroblasts; IV, orthochromatic erythroblasts and reticulocytes; V, retics and RBC). ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ns, P > .05. HCT, hematocrit; HGB, hemoglobin; MCV, mean corpuscular volume; ns, not significant; RBC, red blood cell; ROS, reactive oxygen species.
After 8 weeks, there was an 8.8% increase in the hemoglobin values and a significant improvement in red cell numbers, hematocrit, and mean corpuscular volume (Figure 3C). The inhibition of heme synthesis by bitopertin allows for improved erythropoiesis, but it restricts heme availability for hemoglobin, resulting in a smaller improvement in hemoglobin in the Rpl11-haploinsufficient mice. This small increase is significant when compared to the decrease observed in control mice (Figure 3A; P < .01). Of note, the mean plasma erythropoietin concentration decreased by 74% (from 2011 to 527 pg/mL), reflecting the improvement in anemia. In addition, bitopertin administration, similar to iron restriction,24 significantly reduced erythroblast heme and reactive oxygen species levels at all stages of differentiation, returning the cytoplasmic levels to those observed in untreated control mice (Figure 3D-E).
The improved in vitro erythroid differentiation of marrow from patients with DBA and RPS19-deficient CD34+ cells and the partial amelioration of anemia in Rpl11-haploinsufficient mice suggest that bitopertin might mitigate anemia in patients with DBA. Erythroid culture media contain dexamethasone.19 This raises the possibilities that bitopertin might improve the corticosteroid response of patients with DBA, allow for steroid taper, or be combined with therapies that act upstream of colony-forming unit erythrocyte. In addition, the dose-response curves and data from Rpl11-haploinsufficient mice suggest that a bitopertin dose significantly lower than that used in the phase 2 and 3 trials of schizophrenia and EPP could be effective, limiting any potential side effect.
Acknowledgments
This research was funded by the National Heart, Lung, and Blood Institute, National Institutes of Health grant HL031823 and a grant from Disc Medicine (J.L.A.); and by the National Heart, Lung, and Blood Institute Division of Intramural Research and a Cooperative Research and Development Agreement with Disc Medicine (C.E.D.).
Authorship
Contribution: R.T.D. and J.L.A. designed the studies; R.T.D., A.D.M., H.C., and S.E.F. performed the experiments; M.W. provided bitopertin and quantitated its serum levels; R.T.D., A.D.M., and H.C. collected and analyzed data; D.J.Y. and C.E.D. provided marrow samples from patients with DBA; R.T.D. and J.L.A. wrote and edited the manuscript; and all authors reviewed and approved the manuscript.
Conflict-of-interest disclosure: M.W. is Vice President and Head of Innovation at Disc Medicine. J.L.A. is a scientific advisor to Disc Medicine. The remaining authors declare no competing financial interests.
Correspondence: Janis L. Abkowitz, Division of Hematology and Oncology, Department of Medicine, University of Washington School of Medicine, Box 357710, 1959 NE Pacific St, Seattle, WA 98195; email: janabk@uw.edu.
References
Author notes
Presented in poster form at the 65th annual meeting of the American Society of Hematology, San Diego, CA, 9 to 12 December 2023.
Original data are available on request from author Raymond T. Doty (rtdoty@uw.edu).

![Bitopertin increases the erythroid expansion of marrow from patients with DBA in culture. (A) Blue fluorescent protein positive sorted CD34+ cells expressing either control (shLuc) or RPS19 short hairpin RNA (shRNA) were cultured for 10 days in differentiation media I without or with 10 nM bitopertin (left). The erythroid precursors were then cultured in an EBI culture with CD14-derived macrophage for an additional 3 days in differentiation media II without or with bitopertin (right). Data are presented as the mean and standard error of the mean of the numbers of cells recovered from 3 independent experiments (control: n = 3, RPS19 shRNA: n = 6 [3 each of 2 independent shRNAs]) after normalizing cell counts to reflect starting each culture with 200 000 cells. (B) Characteristics of marrow samples from patients with DBA and control subjects used in studies. (C) Representative flow cytometry analyses of cryopreserved marrow mononuclear cells from a patient with DBA (D2) and a control subject (N1) before and after 3 days in erythroid culture show that erythroid differentiation in patients with DBA fails in vivo at the colony-forming unit erythrocyte and/or proerythroblast stage when CD71 is upregulated, but in vitro culture allows continued differentiation. (D) A representative time course showing the total cell expansion and CD71+ cell expansion of marrow cells from normal (N1) and a patient with DBA (D1) cultured without or with bitopertin. (E) Total cell expansion or expansion of CD71+ cells in marrow cultures from control subjects and patients with DBA for 10 days in differentiation media I without or with bitopertin. Cell counts from each sample were normalized to the culture without bitopertin and presented as a percent of control (n = 4). ∗P < .05; ∗∗∗P < .001; ∗∗∗∗P < .0001; ns, P > .05. ns, not significant.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodrci/1/2/10.1016_j.brci.2025.100010/5/m_brci_rci-2025-000122-gr1.jpeg?Expires=1769103082&Signature=ljKRPJjJwiXmXrkyY73JTTLv31gG4yUv80iFWrPp3AYNHLtvm7kiXWUYYiJ6pm7nLeh-y7VZAQd~uaOe3~BX1gzqzMtI1VGJOlSQRceOlGaS4bW2HUSCzaFbdSdwvpnmqfdGXWXbGenMiEDcNcnVW~~KXOGra0Ksa1o4-aMDyXmxT5j3vsUO1Xbiq0mKo6-sqgSErVotoPg7GJ3lWu-tmqe9Fu9pPLEnunzb~ipBqYJWey8zV6i8QTl~b1XLE6dimwBbAn7jc4MNIU9Jl0f6OWt8Gg0yO6euP47HQzfrigy5X~s~yRNdlCVZzvueuqarSlbPh~4Ua7hp-GVa~tqDHw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)