TO THE EDITOR:
Sickle cell disease (SCD) is a hemoglobinopathy caused by a β-globin gene mutation, leading to sickle-shaped red blood cells that are prone to hemolysis.1 This results in severe complications such as anemia, acute chest syndrome, bone disease, and increased infection risk, significantly affecting quality of life.2 The number of newborns with SCD is projected to exceed 400 000 by 2050, highlighting the need for better interventions. Hematopoietic stem cell transplantation (HSCT) is a leading curative treatment, improving quality of life,3-5 but chronic pain remains a significant burden6 even after HSCT, leading to frequent hospitalizations and costly care. Genetic and environmental factors, including trauma, infection, and stress, contribute to ongoing pain.7-9
The American Academy of Pain Medicine guidelines define chronic pain in SCD as lasting >3 months and affecting the musculoskeletal system, distinguishing it from acute vaso-occlusive pain.10 Understanding these pain characteristics aids clinical management. However, chronic pain after HSCT remains poorly understood, potentially linked to irreversible tissue damage, causing nociceptive and inflammatory changes. Identifying risk factors for chronic pain could improve outcomes for these patients.
At our institution, we assess cardiac, respiratory, and psychosocial health before HSCT. We found that patients with chronic pain are at higher risk for severe pain during and after HSCT, leading to longer hospital stays and poor pain control. To optimize care, we plan to create a checklist in collaboration with referring institutions to identify pain-prone patients before HSCT. By addressing pain risk factors early, we aim to improve pain management, reduce hospitalizations, and enhance patient outcomes and cost-effectiveness.
This was an institutional review board–exempt retrospective medical record review of 89 patients, aged 0 to 21 years, who underwent HSCT for SCD at Children’s National Hospital between 10 October 1996 and 31 March 2023. All analyses were performed using R version 4.3.1.
Descriptive statistics, including medians and 25th and 75th percentiles, were computed to describe continuous variables. Pain scores were obtained from the Faces Pain Scale or Numeric Rating Scale. The mean of 3 scores was used at each time point. Percentages were used to describe items with categorical and ordinal values. Barnard exact tests11 for differences of proportions, χ2 for contingency tables to test for multiple proportions differences, and the Mann-Whitney U test for continuous variables were used for general and marginal comparison of each outcome and covariates.
The main analysis involved describing the distributions of 3 outcomes using generalized linear models (GLMs) based on selected modifiers. Specifically, we used GLM with a log-gamma response and zero-inflated terms for “maximum pain score after HSCT” and linear logistic regression for “continued outpatient physical therapy requirement” and “diagnosis of chronic pain after HSCT.” Zero-inflated terms were applied to the first outcome due to a large proportion of zero pain scores, which required estimating the odds of no pain before modeling positive pain scores and their relationship with key modifiers.
Candidate models for each outcome were derived using GLM lasso regression12-14 and cross-validation. These models were then adjusted based on subject matter expertise to produce the final models (supplemental Table 1). For binary outcomes, we calculated odds ratios (ORs) and 95% confidence intervals (CIs) for all predictors. For “maximum pain score after HSCT,” we estimated conditional rate ratios (RRs) for positive scores and the ORs for scoring exactly 0, with corresponding 95% CI.
Demographic data comparing patients with chronic pain after HSCT and those without are listed in Table 1. Organic causes of pain were evaluated by diagnosing avascular necrosis on magnetic resonance imaging or cholelithiasis on abdominal ultrasound. The following variables were not included in our model: graft type, sex, conditioning regimen, morphine equivalent dose per day in the year preceding HSCT, graft failure, and in-house vs outside institution referral. This was due to multicollinearity with selected predictors (all variables) and/or insufficient variability (all variables), given that the selected predictors were already in the model. Given the low incidence of organic causes of pain, such as avascular necrosis and/or cholelithiasis, we were unable to evaluate this as a predictor in our model. There were no deaths in this patient cohort.
Demographic data comparing patients with chronic pain after HSCT vs those without chronic pain after HSCT
| Demographics . | Chronic pain after HSCT (n = 9) . | Absence of chronic pain after HSCT (n = 78) . | All patients (N = 87) . | P value . |
|---|---|---|---|---|
| Age, median (Q1-Q3), y | 13.0 (13.0-17.0) | 8.0 (5.0-13.8) | 10.0 (5.0-14.0) | .0086 |
| Sex, n (%) | ||||
| Female | 7 (77.8) | 26 (33.3) | 33 (37.9) | .012 |
| Male | 2 (22.2) | 52 (66.7) | 54 (62.1) | |
| Donor type, n (%) | ||||
| Haplo | 3 (33.3) | 11 (14.1) | 14 (16.1) | .3 |
| MRD | 5 (55.6) | 59 (75.6) | 64 (73.6) | |
| MUD | 1 (11.1) | 8 (10.3) | 9 (10.3) | |
| BMT source, n (%) | ||||
| BM | 6 (66.7) | 39 (50.0) | 45 (51.7) | .33 |
| PBSC | 3 (33.3) | 22 (28.2) | 25 (28.7) | |
| Cord | 0 (0.0) | 17 (21.8) | 17 (19.5) | |
| Referral type, n (%) | ||||
| In house | 5 (55.6) | 49 (62.8) | 54 (62.1) | .73 |
| Outside | 4 (44.4) | 29 (37.2) | 33 (37.9) | |
| Post-HSCT pain regimen, n (%) | ||||
| Long-acting opioid | 3 (33.3) | 1 (1.3) | 4 (4.6) | <.001 |
| Medium | 2 (22.2) | 2 (2.6) | 4 (4.6) | |
| None | 2 (22.2) | 57 (73.1) | 59 (67.8) | |
| High | 0 (0.0) | 2 (2.6) | 2 (2.3) | |
| Conditioning regimen, n (%) | ||||
| Myeloablative | 3 (33.3) | 42 (53.8) | 45 (51.7) | .098 |
| Nonmyeloablative | 5 (55.6) | 18 (23.1) | 23 (26.4) | |
| RI | 1 (11.1) | 18 (23.1) | 19 (21.8) | |
| No. of pain admissions per year, n (%) | ||||
| 0 | 1 (11.1) | 64 (82.1) | 65 (74.7) | <.001 |
| 1 | 1 (11.1) | 9 (11.5) | 10 (11.5) | |
| ≥2 | 5 (55.6) | 4 (5.1) | 9 (10.3) | |
| Missing | 2 (22.2) | 1 (1.3) | 3 (3.4) | |
| Mental health diagnosis, n (%) | ||||
| Mood disorder | 4 (44.4) | 1 (1.3) | 5 (5.7) | <.001 |
| None | 3 (33.3) | 72 (92.3) | 75 (86.2) | |
| ADHD | 2 (22.2) | 4 (5.1) | 6 (6.9) | |
| Missing | 0 (0) | 1 (1.3) | 1 (1.1) | |
| Pre-HSCT MMEs/d, n (%) | ||||
| High | 3 (33.3) | 0 (0.0) | 3 (3.4) | <.001 |
| Low | 1 (11.1) | 7 (9.0) | 8 (9.2) | |
| None | 1 (11.1) | 66 (84.6) | 67 (77.0) | |
| Unknown | 3 (33.3) | 4 (5.1) | 7 (8.0) | |
| Very high | 1 (11.1) | 1 (1.3) | 2 (2.3) | |
| Post-HSCT pain score >0, median (Q1-Q3) | 7.0 (4.0-8.0) | 0.0 (0.0-0.0) | 0.0 (0.0-0.0) | <.001 |
| PT evaluation at discharge, n (%) | ||||
| Continued outpatient needs | 7 (77.8) | 21 (26.9) | 28 (32.2) | .049 |
| No further needs | 2 (22.2) | 56 (71.8) | 58 (66.7) | |
| Unknown | 0 (0.0) | 1 (1.3) | 1 (1.1) | |
| Pre-HSCT pain score >0, median (Q1-Q3) | 4.0 (0.0-7.0) | 0.0 (0.0-0.0) | 0.0 (0.0-0.0) | <.001 |
| Pre-HSCT diagnosis of chronic pain, n (%) | ||||
| No | 5 (55.6) | 77 (98.7) | 82 (94.3) | <.001 |
| Yes | 4 (44.4) | 1 (1.3) | 5 (5.7) |
| Demographics . | Chronic pain after HSCT (n = 9) . | Absence of chronic pain after HSCT (n = 78) . | All patients (N = 87) . | P value . |
|---|---|---|---|---|
| Age, median (Q1-Q3), y | 13.0 (13.0-17.0) | 8.0 (5.0-13.8) | 10.0 (5.0-14.0) | .0086 |
| Sex, n (%) | ||||
| Female | 7 (77.8) | 26 (33.3) | 33 (37.9) | .012 |
| Male | 2 (22.2) | 52 (66.7) | 54 (62.1) | |
| Donor type, n (%) | ||||
| Haplo | 3 (33.3) | 11 (14.1) | 14 (16.1) | .3 |
| MRD | 5 (55.6) | 59 (75.6) | 64 (73.6) | |
| MUD | 1 (11.1) | 8 (10.3) | 9 (10.3) | |
| BMT source, n (%) | ||||
| BM | 6 (66.7) | 39 (50.0) | 45 (51.7) | .33 |
| PBSC | 3 (33.3) | 22 (28.2) | 25 (28.7) | |
| Cord | 0 (0.0) | 17 (21.8) | 17 (19.5) | |
| Referral type, n (%) | ||||
| In house | 5 (55.6) | 49 (62.8) | 54 (62.1) | .73 |
| Outside | 4 (44.4) | 29 (37.2) | 33 (37.9) | |
| Post-HSCT pain regimen, n (%) | ||||
| Long-acting opioid | 3 (33.3) | 1 (1.3) | 4 (4.6) | <.001 |
| Medium | 2 (22.2) | 2 (2.6) | 4 (4.6) | |
| None | 2 (22.2) | 57 (73.1) | 59 (67.8) | |
| High | 0 (0.0) | 2 (2.6) | 2 (2.3) | |
| Conditioning regimen, n (%) | ||||
| Myeloablative | 3 (33.3) | 42 (53.8) | 45 (51.7) | .098 |
| Nonmyeloablative | 5 (55.6) | 18 (23.1) | 23 (26.4) | |
| RI | 1 (11.1) | 18 (23.1) | 19 (21.8) | |
| No. of pain admissions per year, n (%) | ||||
| 0 | 1 (11.1) | 64 (82.1) | 65 (74.7) | <.001 |
| 1 | 1 (11.1) | 9 (11.5) | 10 (11.5) | |
| ≥2 | 5 (55.6) | 4 (5.1) | 9 (10.3) | |
| Missing | 2 (22.2) | 1 (1.3) | 3 (3.4) | |
| Mental health diagnosis, n (%) | ||||
| Mood disorder | 4 (44.4) | 1 (1.3) | 5 (5.7) | <.001 |
| None | 3 (33.3) | 72 (92.3) | 75 (86.2) | |
| ADHD | 2 (22.2) | 4 (5.1) | 6 (6.9) | |
| Missing | 0 (0) | 1 (1.3) | 1 (1.1) | |
| Pre-HSCT MMEs/d, n (%) | ||||
| High | 3 (33.3) | 0 (0.0) | 3 (3.4) | <.001 |
| Low | 1 (11.1) | 7 (9.0) | 8 (9.2) | |
| None | 1 (11.1) | 66 (84.6) | 67 (77.0) | |
| Unknown | 3 (33.3) | 4 (5.1) | 7 (8.0) | |
| Very high | 1 (11.1) | 1 (1.3) | 2 (2.3) | |
| Post-HSCT pain score >0, median (Q1-Q3) | 7.0 (4.0-8.0) | 0.0 (0.0-0.0) | 0.0 (0.0-0.0) | <.001 |
| PT evaluation at discharge, n (%) | ||||
| Continued outpatient needs | 7 (77.8) | 21 (26.9) | 28 (32.2) | .049 |
| No further needs | 2 (22.2) | 56 (71.8) | 58 (66.7) | |
| Unknown | 0 (0.0) | 1 (1.3) | 1 (1.1) | |
| Pre-HSCT pain score >0, median (Q1-Q3) | 4.0 (0.0-7.0) | 0.0 (0.0-0.0) | 0.0 (0.0-0.0) | <.001 |
| Pre-HSCT diagnosis of chronic pain, n (%) | ||||
| No | 5 (55.6) | 77 (98.7) | 82 (94.3) | <.001 |
| Yes | 4 (44.4) | 1 (1.3) | 5 (5.7) |
∗∗P < .05; ∗∗∗P < .01.
ADHD, attention-deficit/hyperactivity disorder; BM, bone marrow; BMT, bone marrow transplant; Haplo, haploidentical; MME, morphine equivalent dose; MRD, matched related donor; MUD, matched unrelated donor; PBSC, peripheral blood stem cell; PT, physical therapy; RI, reduced intensity; Q1, 25th percentile.
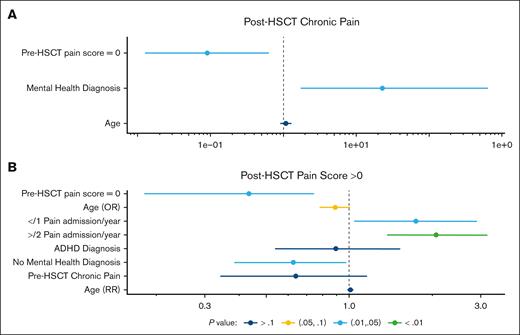
When looking at predictors leading to lower chance of chronic pain after HSCT, we found that patients with a pre-HSCT pain score of 0 have lower odds of chronic pain after HSCT than those with a pre-HSCT pain score >0 (OR, 0.1; 95% CI, 0.0-0.6; P = .02; Figure 1A). Pain scores after transplant were obtained by looking at pain scores on the day of discharge from transplant hospitalization.
Forest plots. (A) OR with 95% CI for development of post-HSCT chronic pain. (B) RR with 95% CI for pain scores >0 after HSCT. ADHD, attention-deficit/hyperactivity disorder.
Forest plots. (A) OR with 95% CI for development of post-HSCT chronic pain. (B) RR with 95% CI for pain scores >0 after HSCT. ADHD, attention-deficit/hyperactivity disorder.
Importantly, patients who were more prone to chronic pain after HSCT included patients with any psychiatric diagnosis. Patients with depression, anxiety, and/or attention-deficit/hyperactivity disorder have a higher odds of chronic pain after HSCT than those without (OR, 22.0; 95% CI, 1.7-645.8; P = .03; Figure 1A).
When looking at the number of pain admissions per year in the year preceding HSCT, patients with ≥2 pain admissions per year have 2.1 times (1.4-3.2) higher maximum pain score after HSCT than those with 0 pain admissions per year (P < .01). Patients who had 1 pain admission per year preceding HSCT have an RR of 1.7 (1.0-2.9), compared to those without any pain admissions, for having post-HSCT pain scores of >0 (P = .4; Figure 1B).
For every 1-unit increase in the pre-HSCT pain score of the patient, the odds of maximum pain score >0 increases by 230% (P = .03) on average. Furthermore, for every 1-year increase in the patient's age, the odds of post-HSCT pain score being >0 increased by 12% (95% CI, 0-28; P = .1; Figure 1B). Supplemental Table 2 shows the ORs and RRs of the outcomes of interest after HSCT.
This study investigates risk factors for chronic pain in pediatric patients after HSCT for SCD. Our findings highlight the complex nature of post-HSCT chronic pain, which persists despite HSCT's curative potential. Preexisting pain, psychiatric conditions, and prior pain admissions are strong predictors of posttransplant pain outcomes. Notably, psychiatric comorbidities such as depression, anxiety, and attention-deficit/hyperactivity disorder emerged as significant risk factors, aligning with prior research on the emotional distress–pain connection.15 This emphasizes the importance of pre-HSCT psychosocial assessments to address these factors.
Our analysis also indicates that patients with a pre-HSCT pain score >0 are more likely to experience chronic pain after transplant, consistent with previous studies suggesting that a history of pain predicts ongoing issues.11,12 Furthermore, frequent pre-HSCT pain admissions (≥2 per year) correlate with higher post-HSCT pain scores, likely due to long-term alterations in pain processing pathways.
Preexisting pain severity significantly predicts posttransplant pain, with an increase in the odds for every 1-unit increase in pre-HSCT pain score. Older age also slightly increased pain risk, possibly due to age-related changes in pain processing and length of SCD duration. These results have clinical implications for pre-HSCT interventions, including targeted pain management, psychosocial support, and preemptive physical therapy.
We recommend incorporating guidelines from the American Academy of Pain Medicine for chronic pain management in pediatric patients with SCD after HSCT. Limitations include the study’s retrospective design, small sample size, and variability in certain predictors. Future research with larger samples is needed to explore chronic pain mechanisms in this population.
In conclusion, our study identifies key predictors of post-HSCT chronic pain, offering insights for improving pretransplant care and long-term pain management in patients with SCD. Future directions include multi-institutional prospective studies to evaluate the impact of the aforementioned targeted interventions.
Acknowledgment: The authors acknowledge the patients included in this study and the entire bone marrow transplant division at Children’s National Hospital.
Contribution: H.B. and C.W. devised the study plan; E.A.T.R. performed statistical analyses; and H.B., E.A.T.R., C.W., and A.M. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Henna Butt, Center for Cancer and Blood Disorders, Children’s National Hospital, 111 Michigan Ave NW, Washington, DC 20010; email: hbutt781@gmail.com.
References
Author notes
Presented in abstract form at the 66th annual meeting of the American Society of Hematology, 7 to 10 December 2024, San Diego, CA.
Data are available on request from the corresponding author, Henna Butt (hbutt781@gmail.com).
The full-text version of this article contains a data supplement.