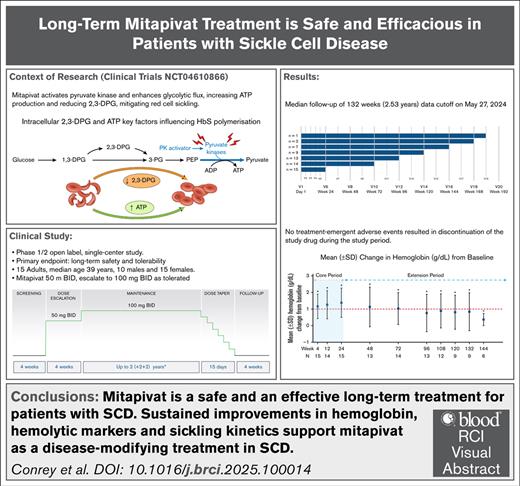
Key Points
Median of 2.53-year follow-up of mitapivat showed favorable safety and tolerability in patients with SCD.
Sustained improvements in Hb, hemolytic markers, and sickling kinetics support mitapivat as a disease-modifying treatment in SCD.
Visual Abstract
In a phase 1 open-label study (ClinicalTrials.gov identifier: NCT04000165), mitapivat, a pyruvate kinase (PK) activator that is approved by the US Food and Drug Administration for treating anemia of PK deficiency, showed promise as a disease-modifying therapy for sickle cell disease (SCD). We now report updated findings from a phase 1/2 study with a median follow-up of 132 weeks (2.53 years), involving 15 patients, 13 from the initial study and 2 new to mitapivat. Patients with hemoglobin SS (HbSS) aged ≥18 years started mitapivat 50 mg twice daily for 4 weeks followed by a dose escalation to 100 mg twice daily for another 20 weeks to complete a 24-week core period. Nine patients continued treatment for >120 weeks, resulting in 1884 patient-weeks of exposure to mitapivat. Common treatment-emergent adverse events (TEAEs) included vaso-occlusive crises (VOCs), decreased hormone levels, arthralgia, cough, and COVID-19 infection. Changes in laboratory values were not clinically significant. Serious TEAEs were mainly VOCs in 10 patients, and lung infections in 3; all VOCs were linked to known triggers. No TEAEs led to discontinuation of mitapivat. Of 15 patients, 14 (93%) had at least a 1 g/dL increase in Hb at some point within the 24-week core period, with a mean increase of 1.38 ± 0.88 g/dL. Improvements in Hb, hemolytic markers, oxygen affinity, sickling kinetics, and the ratio of adenosine triphosphate to 2,3-diphosphoglycerate were sustained during the extension period. These findings suggest that long-term mitapivat is safe and effective for patients with SCD, warranting further investigation in the ongoing phase 3 study (RISE UP; ClinicalTrials.gov identifier: NCT05031780). This trial was registered at www.ClinicalTrials.gov as #NCT04610866.
Introduction
Sickle cell disease (SCD) is an inherited blood disorder caused by a mutation in the β-hemoglobin (β-Hb) gene, leading to the production of abnormal HbS. HbS polymerizes upon deoxygenation, causing red blood cell (RBC) deformation (“sickling”) and stiffening that can cause vaso-occlusion, recurrent episodes of acute pain, and chronic hemolytic anemia that underlie the cumulative organ damage and premature death.1 Two key factors influencing sickling are intracellular levels of 2,3-diphosphoglycerate (2,3-DPG) and adenosine triphosphate (ATP).2-4 2,3-DPG preferentially binds to and stabilizes the polymerizing T conformation of HbS, thus promoting sickling, whereas ATP depletion leads to RBC dehydration and increased sickling.2-4
RBCs rely solely on glycolysis for ATP, which is essential for all energy-dependent activities and cellular integrity,5 with pyruvate kinase (PK) playing a crucial role.6,7 Decreased PK activity reduces ATP and increases 2,3-DPG, an upstream glycolytic intermediate,8 exacerbating sickling. Therefore, activating PK presents a promising treatment strategy for hemolytic anemias such as SCD by boosting ATP9 and lowering 2,3-DPG.10
Mitapivat, an oral PK activator taken twice daily, enhances glycolytic flux, reducing 2,3-DPG and increasing ATP. In 2022, mitapivat was approved in the United States by the US Food and Drug Administration for the treatment of hemolytic anemia in adults with PK deficiency, and in the European Union by the European Medicines Agency and in Great Britain by the Medicines and Healthcare products Regulatory Agency for the treatment of PK deficiency in adults. However, mitapivat also activates wild-type PK in SCD. A phase 1 study (ClinicalTrials.gov identifier: NCT04000165)11 demonstrated mitapivat’s safety and potential efficacy in patients with SCD, showing increased ATP, decreased 2,3-DPG, improved hematologic parameters, enhanced oxygen affinity, and reduced sickling. Other studies confirmed the safety of activating endogenous PK in patients with SCD with similar improvements in hematologic parameters, increased ATP:2,3-DPG ratio, Hb level, and oxygen affinity, and reduced hemolytic markers, in keeping with its mechanism.12-15 Results from the 12-week double-blind, randomized, placebo-controlled phase 2 portion of the RISE UP trial (ClinicalTrials.gov identifier: NCT05031780) confirmed these findings and favored proceeding with mitapivat 100 mg twice daily as the dose in the phase 3 portion of the trial.14 However, the studies to date are limited by duration of study.
Here, we report an extension of our phase 1 dose-escalation study that provides added value in the extended duration of follow-up complementing the phase 2 ESTIMATE (NL8517)13 and the phase 2 portion of RISE UP (ClinicalTrials.gov identifier: NCT05031780)14 studies. We focus on the long-term safety and efficacy of a stable mitapivat dose in patients with SCD.
Methods
Study design, participants, and end points
Details on study design, eligibility criteria, and analytical methodology are provided in the supplemental Methods.
Patients who completed the dose-escalation study of mitapivat (ClinicalTrials.gov identifier: NCT04000165) were invited to enroll on this phase 2 study after completing drug taper and a 4-week post–end-of-study period, ensuring a minimum of an 8-week washout period. Patients who declined or were unable to participate in the extension study were replaced by new patients who had not previously been exposed to mitapivat. This investigator-initiated, open-label study was approved by the National Heart, Lung, and Blood Institute institutional review board and was registered at www.ClinicalTrials.gov (identifier: NCT04610866). The study was conducted in accordance with the principles of the Declaration of Helsinki and good clinical practice guidelines. Trial data were collected and analyzed by authors employed by the National Heart, Lung, and Blood Institute, and the data reviewed on an ongoing basis by the National Institutes of Health (NIH) data and safety monitoring board. The study drug was provided by Agios Pharmaceuticals, Inc, Cambridge, MA.
A total of 15 patients (2 mitapivat naïve), aged ≥18 years with a confirmed diagnosis of SCD (HbSS), and with no recent blood transfusions or recent changes to hydroxyurea (HU) or l-glutamine and had adequate organ function as defined by intact renal and hepatic function without significant cytopenias, were enrolled. Patients with documented PK deficiency or screening Hb of ≥11 g/dL, and those on voxelotor, crizanlizumab, and/or hematopoietic stimulating agents were excluded. Women of reproductive potential were required to use 2 forms of contraception for the duration of the study (see supplemental Materials for full list of inclusion and exclusion criteria).
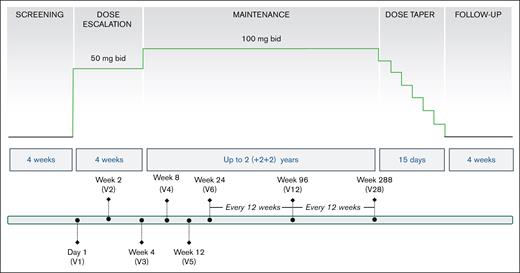
All patients began mitapivat at 50 mg twice daily, escalating after 4 weeks to the maintenance dose of 100 mg twice daily unless there was a rapid Hb rise of ≥2 g/dL, the maximum Hb level of 12.5 g/dL had been reached, or dose increase to 100 mg twice daily was not tolerated because of side effects. Dose adjustments were performed at any time at the discretion of the principal investigator (PI) for any concerns, including those of safety and/or tolerability. Patients who completed 48 weeks of treatment were offered the option of continued treatment for up to an additional 5 years (Figure 1).
Design of mitapivat extension study in SCD. This is a nonrandomized, open-label study, which included 15 patients with HbSS: 13 patients participated and completed the previous phase 1 dose-escalation study, and 2 patients were mitapivat naïve. After screening, all patients started mitapivat at 50 mg twice daily, escalating after 4 weeks to 100 mg twice daily, unless there was a rapid Hb increase of ≥2 g/dL), the maximum Hb level of 12.5 g/dL had been reached, or not tolerated. After completion of the 24-week core period, patients had an option to continue on mitapivat treatment in the extension period for up to 6 years. Study visits were conducted every 2 weeks (×2), 4 weeks (×2), and then every 12 weeks for the first 2 years, and will continue every 24 weeks for the remaining 4 years. The primary end point was long-term safety and tolerability, in conjunction with changes in clinical laboratory values. Secondary end points included assessment of Hb response and changes in hemolytic markers and how well the Hb response was sustained; pharmacokinetic and pharmacodynamic parameters; p50, a measure of oxygen affinity; and t50, a measure of HbS polymerization kinetics.
Design of mitapivat extension study in SCD. This is a nonrandomized, open-label study, which included 15 patients with HbSS: 13 patients participated and completed the previous phase 1 dose-escalation study, and 2 patients were mitapivat naïve. After screening, all patients started mitapivat at 50 mg twice daily, escalating after 4 weeks to 100 mg twice daily, unless there was a rapid Hb increase of ≥2 g/dL), the maximum Hb level of 12.5 g/dL had been reached, or not tolerated. After completion of the 24-week core period, patients had an option to continue on mitapivat treatment in the extension period for up to 6 years. Study visits were conducted every 2 weeks (×2), 4 weeks (×2), and then every 12 weeks for the first 2 years, and will continue every 24 weeks for the remaining 4 years. The primary end point was long-term safety and tolerability, in conjunction with changes in clinical laboratory values. Secondary end points included assessment of Hb response and changes in hemolytic markers and how well the Hb response was sustained; pharmacokinetic and pharmacodynamic parameters; p50, a measure of oxygen affinity; and t50, a measure of HbS polymerization kinetics.
The primary end points were safety, evaluated by the frequency and severity of adverse events (AEs), graded according to the Common Terminology Criteria for Adverse Events, version 5.0 (National Cancer Institute, Bethesda, MD). The main secondary end points included efficacy of treatment with mitapivat evaluated by: (1) Hb response as defined by a ≥1 g/dL increase in Hb at any time point within the 24-week core period compared with baseline and, whether sustained (if the increase was maintained for at least 2 separate time points); (2) changes in markers of hemolysis that include reticulocyte count, lactate dehydrogenase, and serum bilirubin; and (3) pharmacodynamics (2,3-DPG and ATP). Exploratory laboratory end points included oxygen affinity (partial pressure of oxygen [p50], defined as the partial pressure of oxygen at which Hb is 50% saturated with oxygen) and sickling kinetics (t50, defined as the time at which 50% of erythrocytes are sickled in response to gradual deoxygenation with nitrogen to 5% oxygen11). Exploratory end points of clinical benefits include annualized rates of hospital days related to SCD, assessments of cardiopulmonary function (echocardiogram and total distance walked in 6 minutes [6MWT]), and changes in patient health-related quality of life (QOL) that were assessed using the Adult Sickle Cell Quality-of-Life Measurement Information System (ASCQ-Me)16 and Patient-Reported Outcomes Measurement Information System (PROMIS)17 short-form (SF) questionnaires focusing on daily functioning and well-being.
Methodology and assessments
Whole-blood levels of ATP and 2,3-DPG were measured using liquid chromatography with tandem mass spectrometry with a lower limit of quantification of 50 μg/mL and corrected for hematocrit when used in analyses.11 Oxygen affinity was measured using p50 obtained using a Hemox Analyzer [TCS Scientific Corp, New Hope, PA]. Sickling kinetics were assessed by t50.
Data were collected from 5 ASCQ-Me SF measures [(1) pain impact, (2) SCD pain episode frequency, (3) pain episode severity, (4) stiffness impact, and (5) sleep impact] and 3 PROMIS SF measures [(1) cognitive function 6a, (2) dyspnea 10a, and (3) fatigue 6a]. Responses were scored using the item response model at baseline and 24, 48, 72, 96, and 144 weeks on mitapivat.
Statistical analysis
The sample size was primarily determined by the restriction to the 15 participants in this study (ie, the number enrolled in the phase 1 study). The probability of detecting at least 1 AE as a function of sample size and true underlying AE rate is provided in supplemental Table 1. Descriptive statistics were calculated for demographic, laboratory, and safety data. For clinical laboratory values, functional status, and cardiopulmonary function, the descriptive statistics including mean, median, 25th percentile, 75th percentile, and means are based on the change from baseline. For analytes (ATP and 2,3-DPG), sickling kinetics (t50 and p50), and QOL surveys, the same descriptive statistics are presented for the percentage change from baseline. Mean change was used for clinical variables to allow for clinical interpretation of the data, whereas mean percent change was used for analytes, sickling kinetics, and QOL surveys, to reflect relative changes. If a patient had a missing baseline value for a specific variable, they were excluded from the analysis for that variable. In addition, during the study, patients were allowed to receive episodic blood transfusions as clinically indicated, but their data were excluded from analysis unless their HbA levels were ≤20%. For assessing statistically significance changes (or percent changes) from baseline, a generalized estimating equation approach was used to accommodate the longitudinal measurements collected from each person. The P values shown for the significance of change or percent change arise from this generalized estimating equation model that included separate parameter estimates for each follow-up period and a robust sandwich estimator for the variance.
All statistical analyses were conducted using R statistical software (version 4.4.2).
Results
Patients
The extension period of the study is ongoing; here, we report data for up to 144 weeks (2.76 years; data cutoff, 27 May 2024) (supplemental Figure 1). Fifteen patients enrolled (December 2020 to April 2022); 13 had participated in the phase 1 study (ClinicalTrials.gov identifier: NCT04000165), and 2 were mitapivat naïve. The mean age was 39 years (range, 25-57); 10 were male, and 11 were on HU (Table 1). All 15 patients completed the core period of 24 weeks; 14 entered the extension period, with 1 patient on 5 mg twice daily, 1 patient on 50 mg twice daily, 2 alternating between 50 and 100 mg twice daily, and the rest on 100 mg twice daily. Mitapivat was dose-reduced from 100 mg twice daily to 50 mg twice daily in 3 patients: 1 each for reports of pruritus, bloating, and insomnia. As treatment-emergent adverse events (TEAEs) resolved, 2 of 3 patients were reescalated to 100 mg twice daily, without recurrence of symptoms. For 1 patient who entered the extension period at 50 mg twice daily, mitapivat dose was later escalated to 100 mg twice daily at 168 weeks at the PI’s discretion (to improve his Hb level as dose of HU had to be reduced for recurrent neutropenia). One patient maintained a stable Hb ranging from 11.5 to 12.3 g/dL on mitapivat 5 mg twice daily; escalation of dose to 20 or 50 mg twice daily led to Hb increase exceeding the protocol cutoff of 12.5 mg/dL. At the cutoff date of 27 May 2024, all 15 patients had completed 24 weeks, 13 patients had completed 96 weeks of mitapivat treatment (supplemental Figure 1), and the median duration of treatment for patients who continued onto the extension period was 132 weeks (2.53 years). Nine patients have received ≥120 weeks of treatment (supplemental Figure 1), with a total of 1884 patient-weeks of drug exposure thus far. Four patients have come off study: 2 patients discontinued because of moving out of the area (1 after 6 months, and the other after 3 years), 1 patient withdrew after 2 years (pregnancy reasons), and 1 was removed after 1.5 years for poor compliance to both study drug and HU, and study protocol (per PI discretion).
Patient demographics and baseline characteristics
| Characteristic . | N = 15 . |
|---|---|
| Mean age (range), y | 39 (25-57) |
| Sex, n (%) | |
| Female | 5 (33.3) |
| Male | 10 (66.7) |
| African or African American race, n (%) | 15 (100) |
| HbSS genotype, n (%) | 15 (100) |
| HU use, n (%) | 11 (73.3) |
| l-Glutamine use, n (%) | 0 (0.0) |
| Baseline laboratory measures | |
| Mean Hb (SD), g/dL | 8.80 (0.99) |
| Mean absolute reticulocyte count (SD), ×103/μL | 217.21 (88.27) |
| Mean total bilirubin (SD), mg/dL | 2.73 (1.43) |
| Mean lactate dehydrogenase (SD), U/L | 536.71 (222.16) |
| Median HbF (25th, 75th percentile), % | 13.80 (7.40, 23.95) |
| Characteristic . | N = 15 . |
|---|---|
| Mean age (range), y | 39 (25-57) |
| Sex, n (%) | |
| Female | 5 (33.3) |
| Male | 10 (66.7) |
| African or African American race, n (%) | 15 (100) |
| HbSS genotype, n (%) | 15 (100) |
| HU use, n (%) | 11 (73.3) |
| l-Glutamine use, n (%) | 0 (0.0) |
| Baseline laboratory measures | |
| Mean Hb (SD), g/dL | 8.80 (0.99) |
| Mean absolute reticulocyte count (SD), ×103/μL | 217.21 (88.27) |
| Mean total bilirubin (SD), mg/dL | 2.73 (1.43) |
| Mean lactate dehydrogenase (SD), U/L | 536.71 (222.16) |
| Median HbF (25th, 75th percentile), % | 13.80 (7.40, 23.95) |
HbF, fetal Hb; SD, standard deviation.
Laboratory data from the start of study until week 144 (2.76 years) were used for this analysis. Echocardiogram, 6MWT, and QOL surveys were evaluated using data up to week 144 to assess long-term trends in clinical outcomes.
Safety and tolerability
Mitapivat was generally well tolerated; no TEAEs resulted in discontinuation of the study drug during the study period. Any and all vaso-occlusive crises (VOCs) that occurred while on study, regardless of attribution, were considered AEs (Table 2). The most common TEAEs (reported in ≥5 patients) were VOCs (n = 10), estradiol decrease (n = 8), estrone decrease (n = 7), testosterone increase (n = 6), arthralgia (n = 5), COVID-19 infection (n = 5), cough (n = 5), fracture (n = 4), and alanine aminotransferase (ALT) increase (n = 4; Table 3). Changes in laboratory values were not clinically significant.
Summary of TEAEs
| . | AEs, n (%), in all patients (N = 15) . |
|---|---|
| At least 1 AE | 15 (100) |
| At least 1 AE of grade ≥3 | 12 (80) |
| At least 1 serious AE | 11 (73.3) |
| At least 1 serious adverse event possibly related to treatment | 3 (20) |
| . | AEs, n (%), in all patients (N = 15) . |
|---|---|
| At least 1 AE | 15 (100) |
| At least 1 AE of grade ≥3 | 12 (80) |
| At least 1 serious AE | 11 (73.3) |
| At least 1 serious adverse event possibly related to treatment | 3 (20) |
Most common TEAEs
| TEAE . | No. of patients with TEAEs (%) . | |
|---|---|---|
| All grades (≥10%) . | Grade ≥3 . | |
| VOC | 10 (66.7) | 10 (66.7) |
| Estradiol decreased | 8 (53.3) | 0 (0) |
| Estrone decreased | 7 (46.7) | 0 (0) |
| Testosterone increased, total | 6 (40) | 0 (0) |
| Arthralgia | 5 (33.3) | 0 (0) |
| Cough | 5 (33.3) | 0 (0) |
| COVID-19 infection | 5 (33.3) | 1 (6.7) |
| ALT increased | 4 (26.7) | 0 (0) |
| Bloating | 4 (26.7) | 0 (0) |
| Influenza-like symptoms | 4 (26.7) | 0 (0) |
| Fracture∗ | 4 (26.7) | 1 (6.7) |
| Lung infection | 4 (26.7) | 4 (26.7) |
| Pain in extremity | 4 (26.7) | 0 (0) |
| Upper respiratory infection | 4 (26.7) | 0 (0) |
| Back pain | 3 (20) | 2 (13.3) |
| CPK increased | 3 (20) | 1 (6.7) |
| Creatinine increased | 3 (20) | 0 (0) |
| Dehydration | 3 (20) | 0 (0) |
| Edema limbs | 3 (20) | 0 (0) |
| Fall | 3 (20) | 0 (0) |
| Headache | 3 (20) | 0 (0) |
| Hypertriglyceridemia | 3 (20) | 0 (0) |
| Insomnia | 3 (20) | 0 (0) |
| Lymph node pain | 3 (20) | 0 (0) |
| Increased appetite | 3 (20) | 0 (0) |
| Osteoporosis | 3 (20) | 0 (0) |
| Pain | 3 (20) | 1 (6.7) |
| Skin ulceration | 3 (20) | 0 (0) |
| Allergic reaction | 2 (13.3) | 0 (0) |
| Anorexia | 2 (13.3) | 0 (0) |
| Belching | 2 (13.3) | 0 (0) |
| Bilirubin increased (direct) | 2 (13.3) | 0 (0) |
| Chest wall pain | 2 (13.3) | 0 (0) |
| Chills | 2 (13.3) | 0 (0) |
| Diarrhea | 2 (13.3) | 0 (0) |
| Dry mouth | 2 (13.3) | 0 (0) |
| Hypomagnesemia | 2 (13.3) | 0 (0) |
| Laceration | 2 (13.3) | 1 (6.7) |
| Nasal congestion | 2 (13.3) | 0 (0) |
| Neutrophil count decreased | 2 (13.3) | 0 (0) |
| Noncardiac chest pain | 2 (13.3) | 0 (0) |
| Productive cough | 2 (13.3) | 0 (0) |
| Superficial thrombophlebitis | 2 (13.3) | 0 (0) |
| Testosterone deficiency | 2 (13.3) | 0 (0) |
| Toothache | 2 (13.3) | 0 (0) |
| Vaccination complication | 2 (13.3) | 0 (0) |
| Vertigo | 2 (13.3) | 0 (0) |
| TEAE . | No. of patients with TEAEs (%) . | |
|---|---|---|
| All grades (≥10%) . | Grade ≥3 . | |
| VOC | 10 (66.7) | 10 (66.7) |
| Estradiol decreased | 8 (53.3) | 0 (0) |
| Estrone decreased | 7 (46.7) | 0 (0) |
| Testosterone increased, total | 6 (40) | 0 (0) |
| Arthralgia | 5 (33.3) | 0 (0) |
| Cough | 5 (33.3) | 0 (0) |
| COVID-19 infection | 5 (33.3) | 1 (6.7) |
| ALT increased | 4 (26.7) | 0 (0) |
| Bloating | 4 (26.7) | 0 (0) |
| Influenza-like symptoms | 4 (26.7) | 0 (0) |
| Fracture∗ | 4 (26.7) | 1 (6.7) |
| Lung infection | 4 (26.7) | 4 (26.7) |
| Pain in extremity | 4 (26.7) | 0 (0) |
| Upper respiratory infection | 4 (26.7) | 0 (0) |
| Back pain | 3 (20) | 2 (13.3) |
| CPK increased | 3 (20) | 1 (6.7) |
| Creatinine increased | 3 (20) | 0 (0) |
| Dehydration | 3 (20) | 0 (0) |
| Edema limbs | 3 (20) | 0 (0) |
| Fall | 3 (20) | 0 (0) |
| Headache | 3 (20) | 0 (0) |
| Hypertriglyceridemia | 3 (20) | 0 (0) |
| Insomnia | 3 (20) | 0 (0) |
| Lymph node pain | 3 (20) | 0 (0) |
| Increased appetite | 3 (20) | 0 (0) |
| Osteoporosis | 3 (20) | 0 (0) |
| Pain | 3 (20) | 1 (6.7) |
| Skin ulceration | 3 (20) | 0 (0) |
| Allergic reaction | 2 (13.3) | 0 (0) |
| Anorexia | 2 (13.3) | 0 (0) |
| Belching | 2 (13.3) | 0 (0) |
| Bilirubin increased (direct) | 2 (13.3) | 0 (0) |
| Chest wall pain | 2 (13.3) | 0 (0) |
| Chills | 2 (13.3) | 0 (0) |
| Diarrhea | 2 (13.3) | 0 (0) |
| Dry mouth | 2 (13.3) | 0 (0) |
| Hypomagnesemia | 2 (13.3) | 0 (0) |
| Laceration | 2 (13.3) | 1 (6.7) |
| Nasal congestion | 2 (13.3) | 0 (0) |
| Neutrophil count decreased | 2 (13.3) | 0 (0) |
| Noncardiac chest pain | 2 (13.3) | 0 (0) |
| Productive cough | 2 (13.3) | 0 (0) |
| Superficial thrombophlebitis | 2 (13.3) | 0 (0) |
| Testosterone deficiency | 2 (13.3) | 0 (0) |
| Toothache | 2 (13.3) | 0 (0) |
| Vaccination complication | 2 (13.3) | 0 (0) |
| Vertigo | 2 (13.3) | 0 (0) |
CPK, creatine phosphokinase.
All fractures were the result of a traumatic event that took place. One fracture was classified as grade 3 because there was also an accompanying laceration at the fracture site that required suturing. Also, of note, 3 of 4 fractures took place in the beginning of the study, just 1 to 2 months after the start of mitapivat.
There were 39 serious TEAEs, 28 of which were VOCs in 10 patients. Of these, 4 VOCs (in 3 patients) were attributed as possibly related to study drug; 1 occurred ∼1 month after study drug initiation, 1 occurred shortly after study drug was tapered off, and the other 2 (both from the same patient) were listed as possibly related because it was over their average annualized number of VOCs reported at enrollment. The remaining VOCs that occurred were within the patients’ reported average annual VOC frequency. All VOCs were linked to known triggers. All other serious TEAEs were deemed unrelated to mitapivat. Aside from these, there were 11 other grade 3 TEAEs in 5 patients, which were all unrelated to study drug (Table 4).
Serious TEAEs
| Grade 3 TEAEs . | No. of events . | No. of patients (%) . |
|---|---|---|
| All | 39 | 11 (73.3) |
| VOC∗ | 28 | 10 (66.7) |
| Lung infection | 3 | 3 (20) |
| Back pain | 1 | 1 (6.7) |
| Hypoxia | 1 | 1 (6.7) |
| Gout flare | 2 | 1 (6.7) |
| Opioid withdrawal | 1 | 1 (6.7) |
| Pain | 1 | 1 (6.7) |
| COVID-19 infection | 1 | 1 (6.7) |
| Vomiting | 1 | 1 (6.7) |
| Grade 3 TEAEs . | No. of events . | No. of patients (%) . |
|---|---|---|
| All | 39 | 11 (73.3) |
| VOC∗ | 28 | 10 (66.7) |
| Lung infection | 3 | 3 (20) |
| Back pain | 1 | 1 (6.7) |
| Hypoxia | 1 | 1 (6.7) |
| Gout flare | 2 | 1 (6.7) |
| Opioid withdrawal | 1 | 1 (6.7) |
| Pain | 1 | 1 (6.7) |
| COVID-19 infection | 1 | 1 (6.7) |
| Vomiting | 1 | 1 (6.7) |
A total of 39 serious TEAEs occurred in 11 patients, of which 28 were VOCs in 10 patients: 1 in 3 patients, 2 in 2 patients, 3 in 2 patients, 4 in 1 patient, 5 in 1 patient, and 6 in 1 patient. Overall, 4 of 16 VOCs in 3 patients were possibly related to study drug (1 during drug escalation, 1 after drug taper). A cluster of 5 VOCs over 10 months in 1 patient could be considered as possibly drug related because the frequency was above his calculated annualized baseline of 3.7 per year. However, these VOCs occurred in a setting of known VOC triggers.
VOC is defined as acute clinical events that have no evident cause other than SCD, including acute episodes of pain requiring treatment with parenteral opioids at a medical facility, acute chest syndrome, hepatic sequestration, splenic sequestration, and priapism.
Hormone abnormalities (decreased estradiol and estrone, increased testosterone) occurred only in the male participants (6/10 men). The mean ± standard deviation changes from baseline were 170.05 ± 205.14 ng/dL for testosterone, −31.19 ± 14.16 pg/mL for estrone, and −10.28 ± 8.72 pg/mL for estradiol in all 10 male participants. The only clinical manifestation appreciated in these participants was increased appetite and were otherwise asymptomatic. To date, there have been 4 AEs for increased appetite (in 3 patients), 3 of which occurred within 1 month of study drug initiation and were transient, lasting for just days, up to 1 month of onset, while on the lower dose tier of 50 mg twice daily. The fourth AE of increased appetite occurred ∼1 year after drug initiation and lasted 2 months (also on 50 mg twice daily dosing). Because the hormone changes were biochemical and not accompanied by symptoms or signs of venous thromboembolism or hyperviscosity, they were considered as not clinically significant. Arthralgia, COVID-19, and cough were deemed unrelated to mitapivat by the investigators.
Fractures occurred in 4 patients during the study period, all of which were traumatic and involved just single digits of the hands or feet. Three of the fractures occurred in patients who had osteopenia at baseline in ≥1 sites (supplemental Figure 6) before initiation of study drug, and in all 3 patients the fractures occurred within just 2 months of starting study drug. The fourth fracture (left 5th digit of the foot) occurred in a patient 2 years after enrollment in the study and who has maintained normal bone density. Mean and individualized longitudinal changes in T-scores and Z-scores while on mitapivat are detailed in supplemental Figures 5-7.
ALT increase met criteria for grade 1 AE in 3 patients, and grade 2 AE in the fourth patient. However, these ALT increases were temporary and resolved by the time of the next laboratory test in all patients. Although the increases in ALT were statistically significant compared with baseline, these were small in magnitude and not clinically significant (2.8-11 U/L; supplemental Table 2). Changes in aspartate aminotransferase were statistically significant for 2 time points, weeks 120 and 144, but were also small in magnitude and not clinically significant (supplemental Table 2).
Efficacy on laboratory endpoints
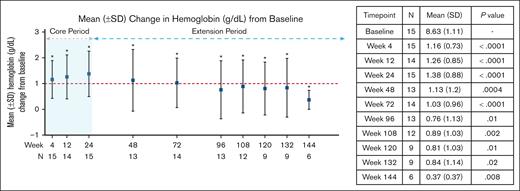
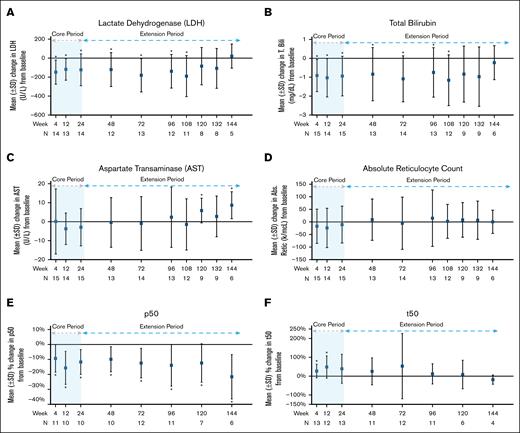
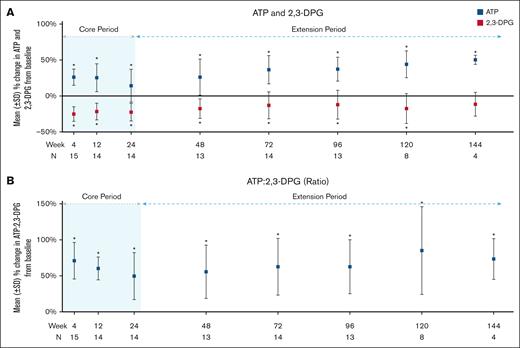
Of 15 patients, 14 (93%) met the secondary endpoint changes of Hb response as defined by at least 1 g/dL increase in Hb at any time point within the 24-week core period compared with baseline, and the increase was maintained for at least 2 separate time points in 12 of 14 patients (85.7%). The mean Hb increase at 24 weeks was 1.38 ± 0.88 g/dL (P < .0001; Table 5; supplemental Table 2). At 120 weeks (n = 9), the mean Hb increase was 0.81 ± 1.03 g/dL (P = .01; Figure 2; Table 5; supplemental Table 2). Changes from baseline in lactate dehydrogenase and total bilirubin at the end of the core period (24 weeks) and 108 weeks revealed significant improvement (Figure 3A-B; Table 5; supplemental Table 2) but no significant changes to aspartate aminotransferase and absolute reticulocyte count (Figure 3C-D; Table 5; supplemental Table 2) were noted. There was a significant reduction in mean percentage change in p50 levels at all evaluated time points. An increase in the mean change of t50 values was also observed throughout 120 weeks, but the increase was not significant after 12 weeks (Figure 3E-F; Table 5; supplemental Table 2). A mean decrease of 22.46% (±12.34%; P < .0001) in 2,3-DPG, increase in ATP of 14.35% (±22.78%; P = .01), and mean increase in ATP:2,3-DPG ratio of 49.73% (±32.68%; P < .0001) were observed at 24 weeks. These changes were maintained for all 3 parameters at the 120-week time point and remained significant (Figure 4; Table 5; supplemental Table 2).
Changes from baseline in hematologic, biochemical, oxygen affinity (p50), sickling kinetics (t50), and pharmacodynamics in response to mitapivat at various longitudinal time points
| . | Baseline . | Week 4 Change from baseline . | Week 12 Change from baseline . | Week 24 Change from baseline . | Week 48 (1 year) Change from baseline . | Week 72 Change from baseline . | Week 96 Change from baseline . | Week 108 Change from baseline . | Week 120 Change from baseline . | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n . | Mean (SD) . | n . | Mean (SD) . | P value . | n . | Mean (SD) . | P value . | n . | Mean (SD) . | P value . | n . | Mean (SD) . | P value . | n . | Mean (SD) . | P value . | n . | Mean (SD) . | P value . | n . | Mean (SD) . | P value . | n . | Mean (SD) . | P value . | |
| Hb (g/dL) | 15 | 8.63 (1.11) | 15 | 1.16 (0.73) | <.0001 | 14 | 1.26 (0.85) | <.0001 | 15 | 1.38 (0.88) | <.0001 | 13 | 1.13 (1.2) | .0004 | 14 | 1.03 (0.96) | <.0001 | 13 | 0.76 (1.13) | .01 | 12 | 0.89 (1.03) | .002 | 9 | 0.81 (1.03) | .01 |
| ARC (×103/μL) | 15 | 217.21 (88.27) | 15 | −17.23 (68.03) | .31 | 14 | −23.68 (78.25) | .24 | 15 | −10.51 (73.37) | .57 | 13 | 8.98 (82.26) | .68 | 14 | −5.17 (103.92) | .85 | 13 | 14.72 (112.07) | .62 | 12 | 2.27 (67.54) | .9 | 9 | 8.24 (68.99) | .70 |
| LDH (U/L) | 14 | 536.71 (222.16) | 14 | −147.93 (127.85) | <.0001 | 13 | −118.23 (113.8) | <.0001 | 14 | −122.86 (167.28) | .004 | 12 | −120.25 (178.83) | .01 | 13 | −180.08 (175.89) | .0001 | 12 | −138.42 (177.54) | .005 | 11 | −189.91 (216.12) | .002 | 8 | −84.50 (197.01) | .19 |
| AST (U/L) | 15 | 39.67 (15.01) | 15 | 0.13 (17.15) | .98 | 14 | −3.71 (8.29) | .08 | 15 | −2.93 (9.84) | .23 | 13 | −0.38 (13.1) | .91 | 14 | −0.93 (14.17) | .8 | 13 | 2.38 (15.89) | .57 | 12 | −1.42 (13.49) | .7 | 9 | 5.89 (6.51) | .004 |
| T. Bili (mg/dL) | 15 | 2.73 (1.43) | 15 | −0.91 (0.85) | <.0001 | 14 | −1.03 (1.01) | <.0001 | 15 | −0.94 (1.05) | .0003 | 13 | −0.84 (1.41) | .03 | 14 | −1.09 (1.22) | .0005 | 13 | −0.75 (1.31) | .03 | 12 | −1.16 (1.34) | .002 | 9 | −0.83 (1.48) | .07 |
| p50 (torr) | 15 | 32.91 (3.44) | 13 | −8.94 (9.59) | .0005 | 14 | −12.78 (12.01) | <.0001 | 13 | −9.37 (10.78) | .001 | 13 | −9.61 (9.59) | .0002 | 14 | −13.56 (10.14) | <.0001 | 13 | −13.67 (13.86) | .0002 | 8 | −13.08 (12.10) | .001 | |||
| t50 (min) | 13 | 159.58 (89.47) | 13 | 26.93 (36.17) | .005 | 12 | 48.56 (59.16) | .003 | 13 | 38.89 (77.21) | .06 | 11 | 25.36 (71.52) | .22 | 12 | 53.17 (173.68) | .27 | 11 | 12.19 (54.01) | .43 | 6 | 8.62 (75.84) | .76 | |||
| 2,3-DPG (μg/mL) | 15 | 16.98 (1.83) | 15 | −25.08 (10.17) | <.0001 | 14 | −21.53 (11.67) | <.0001 | 14 | −22.46 (12.34) | <.0001 | 13 | −17.55 (13.69) | <.0001 | 14 | −12.95 (18.75) | .007 | 13 | −12.12 (20.09) | .02 | 8 | −17.46 (20.78) | .01 | |||
| ATP (μg/mL) | 15 | 7.93 (1.03) | 15 | 26.17 (11.27) | <.0001 | 14 | 25.48 (19.36) | <.0001 | 14 | 14.35 (22.78) | .01 | 13 | 26.15 (25.22) | <.0001 | 14 | 36.6 (19.61) | <.0001 | 13 | 37.25 (16.71) | <.0001 | 8 | 44.09 (18.61) | <.0001 | |||
| ATP:2,3-DPG ratio | 15 | 0.47 (0.06) | 15 | 71.07 (25.41) | <.0001 | 14 | 60.34 (15.89) | <.0001 | 14 | 49.73 (32.68) | <.0001 | 13 | 55.71 (36.98) | <.0001 | 14 | 62.66 (39.38) | <.0001 | 13 | 62.7 (37.46) | <.0001 | 8 | 85.22 (60.92) | <.0001 | |||
| . | Baseline . | Week 4 Change from baseline . | Week 12 Change from baseline . | Week 24 Change from baseline . | Week 48 (1 year) Change from baseline . | Week 72 Change from baseline . | Week 96 Change from baseline . | Week 108 Change from baseline . | Week 120 Change from baseline . | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n . | Mean (SD) . | n . | Mean (SD) . | P value . | n . | Mean (SD) . | P value . | n . | Mean (SD) . | P value . | n . | Mean (SD) . | P value . | n . | Mean (SD) . | P value . | n . | Mean (SD) . | P value . | n . | Mean (SD) . | P value . | n . | Mean (SD) . | P value . | |
| Hb (g/dL) | 15 | 8.63 (1.11) | 15 | 1.16 (0.73) | <.0001 | 14 | 1.26 (0.85) | <.0001 | 15 | 1.38 (0.88) | <.0001 | 13 | 1.13 (1.2) | .0004 | 14 | 1.03 (0.96) | <.0001 | 13 | 0.76 (1.13) | .01 | 12 | 0.89 (1.03) | .002 | 9 | 0.81 (1.03) | .01 |
| ARC (×103/μL) | 15 | 217.21 (88.27) | 15 | −17.23 (68.03) | .31 | 14 | −23.68 (78.25) | .24 | 15 | −10.51 (73.37) | .57 | 13 | 8.98 (82.26) | .68 | 14 | −5.17 (103.92) | .85 | 13 | 14.72 (112.07) | .62 | 12 | 2.27 (67.54) | .9 | 9 | 8.24 (68.99) | .70 |
| LDH (U/L) | 14 | 536.71 (222.16) | 14 | −147.93 (127.85) | <.0001 | 13 | −118.23 (113.8) | <.0001 | 14 | −122.86 (167.28) | .004 | 12 | −120.25 (178.83) | .01 | 13 | −180.08 (175.89) | .0001 | 12 | −138.42 (177.54) | .005 | 11 | −189.91 (216.12) | .002 | 8 | −84.50 (197.01) | .19 |
| AST (U/L) | 15 | 39.67 (15.01) | 15 | 0.13 (17.15) | .98 | 14 | −3.71 (8.29) | .08 | 15 | −2.93 (9.84) | .23 | 13 | −0.38 (13.1) | .91 | 14 | −0.93 (14.17) | .8 | 13 | 2.38 (15.89) | .57 | 12 | −1.42 (13.49) | .7 | 9 | 5.89 (6.51) | .004 |
| T. Bili (mg/dL) | 15 | 2.73 (1.43) | 15 | −0.91 (0.85) | <.0001 | 14 | −1.03 (1.01) | <.0001 | 15 | −0.94 (1.05) | .0003 | 13 | −0.84 (1.41) | .03 | 14 | −1.09 (1.22) | .0005 | 13 | −0.75 (1.31) | .03 | 12 | −1.16 (1.34) | .002 | 9 | −0.83 (1.48) | .07 |
| p50 (torr) | 15 | 32.91 (3.44) | 13 | −8.94 (9.59) | .0005 | 14 | −12.78 (12.01) | <.0001 | 13 | −9.37 (10.78) | .001 | 13 | −9.61 (9.59) | .0002 | 14 | −13.56 (10.14) | <.0001 | 13 | −13.67 (13.86) | .0002 | 8 | −13.08 (12.10) | .001 | |||
| t50 (min) | 13 | 159.58 (89.47) | 13 | 26.93 (36.17) | .005 | 12 | 48.56 (59.16) | .003 | 13 | 38.89 (77.21) | .06 | 11 | 25.36 (71.52) | .22 | 12 | 53.17 (173.68) | .27 | 11 | 12.19 (54.01) | .43 | 6 | 8.62 (75.84) | .76 | |||
| 2,3-DPG (μg/mL) | 15 | 16.98 (1.83) | 15 | −25.08 (10.17) | <.0001 | 14 | −21.53 (11.67) | <.0001 | 14 | −22.46 (12.34) | <.0001 | 13 | −17.55 (13.69) | <.0001 | 14 | −12.95 (18.75) | .007 | 13 | −12.12 (20.09) | .02 | 8 | −17.46 (20.78) | .01 | |||
| ATP (μg/mL) | 15 | 7.93 (1.03) | 15 | 26.17 (11.27) | <.0001 | 14 | 25.48 (19.36) | <.0001 | 14 | 14.35 (22.78) | .01 | 13 | 26.15 (25.22) | <.0001 | 14 | 36.6 (19.61) | <.0001 | 13 | 37.25 (16.71) | <.0001 | 8 | 44.09 (18.61) | <.0001 | |||
| ATP:2,3-DPG ratio | 15 | 0.47 (0.06) | 15 | 71.07 (25.41) | <.0001 | 14 | 60.34 (15.89) | <.0001 | 14 | 49.73 (32.68) | <.0001 | 13 | 55.71 (36.98) | <.0001 | 14 | 62.66 (39.38) | <.0001 | 13 | 62.7 (37.46) | <.0001 | 8 | 85.22 (60.92) | <.0001 | |||
Mean change was calculated from baseline (before drug initiation) and was reported as change for clinical laboratory measures (first 5 rows) and as percent change for p50 and t50, 2,3-DPG, ATP, and ATP:2,3-DPG ratio (last 5 rows). 2,3-DPG and ATP levels used in analyses were corrected for hematocrit. After week 96, collection of exploratory markers (p50, t50, 2,3-DPG, and ATP) took place less often, every 6 months. For data beyond week 120 refer to Supplemental Table 2.
ARC, absolute reticulocyte count; AST, aspartate aminotransferase; LDH, lactate dehydrogenase; T. Bili, total bilirubin.
Hb response in study participants. An asterisk indicates a significant change from baseline at that time point. The P values are included in the inset table. SD, standard deviation.
Hb response in study participants. An asterisk indicates a significant change from baseline at that time point. The P values are included in the inset table. SD, standard deviation.
Change from baseline in study participants for lactate dehydrogenase (A), total bilirubin (B), aspartate transaminase (C), absolute reticulocyte count (D), p50 (E), and t50 (F). An asterisk indicates a significant change from baseline at that time point. The P values are in supplemental Table 2. T. Bili, total bilirubin.
Change from baseline in study participants for lactate dehydrogenase (A), total bilirubin (B), aspartate transaminase (C), absolute reticulocyte count (D), p50 (E), and t50 (F). An asterisk indicates a significant change from baseline at that time point. The P values are in supplemental Table 2. T. Bili, total bilirubin.
Change from baseline in study participants for ATP and 2,3-DPG (A) and ATP:2,3-DPG ratio (B). An asterisk indicates a significant change from baseline at that time point. The P values are in supplemental Table 2.
Change from baseline in study participants for ATP and 2,3-DPG (A) and ATP:2,3-DPG ratio (B). An asterisk indicates a significant change from baseline at that time point. The P values are in supplemental Table 2.
Efficacy on clinical end points and health-related QOL surveys
Cardiopulmonary function was assessed based on echocardiogram changes in cardiac index, right ventricular systolic pressure, and tricuspid jet velocity; and changes in 6MWT distance. These clinical end point variables were evaluated by calculating the change from baseline for each variable at each of the time points evaluated (supplemental Table 2). Overall, decreases were noted for cardiac index, right ventricular systolic pressure, and tricuspid jet velocity, but none were statistically significant. Increases in 6MWT (except at 24 weeks) were observed, but these changes were not significant (supplemental Table 2). In ASCQ-Me surveys, higher scores for pain frequency and severity indicate more suffering but better health for stiffness, pain impact, and sleep.17 In PROMIS surveys, lower scores for fatigue and dyspnea, and higher scores for cognition, favor better health.17 None of the components of these surveys showed significant percentage changes from baseline, with the exception of pain severity at 48 weeks (supplemental Table 2). To illustrate the interpatient variability in the clinical end points, we calculated the percentage change from baseline at each time point for each patient and then averaged the percentage changes across all time points for each individual to produce a waterfall plot for each variable. Interpatient variability in clinical parameters is clearly illustrated in these waterfall plots (supplemental Figures 2-4).
Discussion
SCD is a global health issue, affecting millions worldwide.18 With improvements in childhood survival, SCD has evolved into a chronic debilitating disorder, but therapeutic options remain limited. Curative therapies (autologous [genetic] and allogeneic hemopoietic stem cell transplant) are currently available to few patients, even in well-resourced countries.19,20 HU remains the mainstay medication for managing and treating SCD.21-23 Although HU is an efficacious antisickling agent, it is not universally effective, and an estimated 30% of patients with SCD cannot tolerate HU or do not experience clinical benefits.24 Thus, there is a huge unmet need to develop more oral drugs accessible to patients worldwide that has prompted an increasing number of agents under clinical development (https://clinicaltrials.gov).
Long-term data from this extended study indicate that a fixed dose of mitapivat is safe and well tolerated in adults with SCD after 2.76 years of follow-up, marking the longest exposure of mitapivat in patients with SCD. With a median follow-up of 132 weeks (2.53 years), mitapivat showed statistically significant, durable, and clinically meaningful improvements in Hb levels that were sustained and accompanied by sustained improvements in markers of hemolysis, although some fluctuations in improvements were observed. There were clinical events that have likely contributed to fluctuations in Hb values over the course of the study. HU dosing was decreased in 5 patients over time for reasons such as recurrent neutropenia and leg ulcerations that were stagnating in the healing process. One patient underwent a total hip revision surgery without the need for blood products ∼1 month before a time point that showed a 1-g/dL drop in his Hb from the previous time point (12.3 to 11.3 g/dL). Another patient had an ∼1-g/dL drop in Hb at 72 weeks from a VOC admission that occurred <1 month before that protocol time point. These changes are likely to affect a study that has only 15 participants.
Nonetheless, the improvements in Hb levels and hemolytic markers occurred within 4 weeks of mitapivat treatment. Mitapivat reduced 2,3-DPG and increased ATP, consistent with its mechanism of action, with expected increase in RBC oxygen affinity and improvements in sickling kinetics.
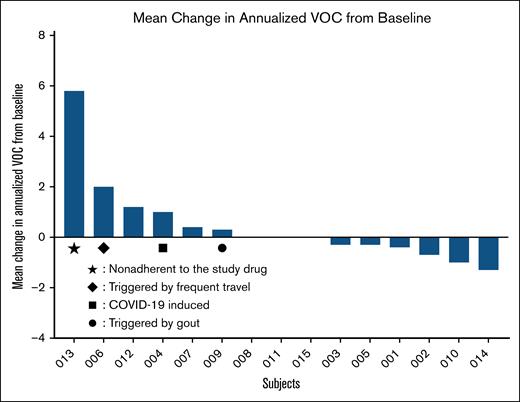
Although the study is a documentation of the longest exposure of patients with SCD to mitapivat, the study’s sample size and variability of self-reported outcomes limited the ability to assess VOCs and QOL measures that are evaluated by questionnaires. VOCs are complex because of their multifactorial nature, influenced by psychological and environmental factors, and often underestimated in health care settings.25 Many patients manage pain at home, so health care use may often underestimate the true pain burden, particularly during the COVID-19 pandemic. Thus, the baseline number of hospital-related admissions before enrolling in the extension study could be an underestimate of VOC events, given that this period overlapped with the COVID-19 pandemic. Once enrolled, patients were encouraged to report pain and seek treatment for severe VOCs at the NIH Clinical Center, potentially increasing observed hospital-related pain days. Furthermore, more than half of adults with SCD also experience chronic pain, and acute-on-chronic pain may have contributed to the observed variability in the response to questionnaires.26 The study was not powered to detect statistically significant changes in sickle pain crises, or end points related to activities of daily living that may be better captured in a larger sample size of participants. Individualized mean change from baseline in annualized VOC clearly illustrates the huge interindividual variation; in particular, increases in VOC in 4 patients should be interpreted in the context of poor compliance with HU and study drug, frequent air travel, COVID-19 infection, and gouty attacks, in 1 patient each (Figure 5).
Individualized mean change from baseline in annualized VOC. Note that increased VOC in 4 patients should be interpreted in the context of poor compliance with HU and study drug, frequent air travel, COVID-19 infection and gouty attacks, in 1 patient each.
Individualized mean change from baseline in annualized VOC. Note that increased VOC in 4 patients should be interpreted in the context of poor compliance with HU and study drug, frequent air travel, COVID-19 infection and gouty attacks, in 1 patient each.
With regard to the 4 fractures that occurred in 4 patients, it should be noted that the NIH Clinical Center measures bone densitometry in 4 anatomical sites: anteroposterior spine, femoral neck, total hip, and radius. Overall, bone mineral density (T-scores and Z-scores) remained stable over time, with the exception of 2 patients. One of these patients had a mean T-score in the osteopenic range at baseline that improved during the study period to the normal range. The other patient’s mean T-score progressed from osteopenia at baseline to osteoporosis (supplemental Figure 5A-B). Individual T-score trajectories according to imaging site can be found in supplemental Figures 6 and 7. None of the patients were taking bisphosphonates. As mentioned earlier, the fractures occurred in 3 of 4 patients with osteopenia before study enrollment early in study course within 2 months of exposure to mitapivat and were induced by trauma. The fourth fracture occurred in a patient 2 years after enrollment in the study and who has maintained normal bone density. Disorders of bone density are underexamined in SCD, and we hope this discussion highlights the need for additional studies and their impact on patients.
A recurring theme in SCD is the enormous interpatient and intrapatient variability in clinical phenotypes and laboratory values. Hence, it is not too surprising to note variability in clinical improvements that are clearly depicted in the individualized waterfall plots (supplemental Figures 2-4). For example, 7 patients had increased mean change in 6MWT distance from baseline but 8 had a decrease (waterfall plot; supplemental Figure 2D). Decreased 6MWT in 1 patient was most likely affected by hip pain due to avascular necrosis that was subsequently resolved by a total hip replacement.
Activating endogenous PK breaks new ground in the therapeutic landscape for SCD via 2 antisickling mechanisms: increases in ATP and decreases in 2,3-DPG.11,13-15,27 Increasing ATP is also a very effective mechanism for treating a broad range of hemolytic anemias including PK deficiency28 caused by mutations in the PKLR gene that encodes PK, and thalassemias (both α and β).29 Recent work from our group provides multiomics and cellular evidence of benefits from increasing ATP beyond improving red cell hydration.30-32
The data reported here show that mitapivat is a safe and an effective long-term treatment for SCD, supporting its potential as a disease-modifying medication and further investigation in the ongoing phase 3 study (RISE UP; ClinicalTrials.gov identifier: NCT05031780). Mitapivat continues to be evaluated in the extension study (NCT04610866).
Acknowledgments
Mitapivat was provided by Agios Pharmaceuticals, Inc, Cambridge, MA. The pyruvate kinase, 2,3-diphosphoglycerate, and adenosine triphosphate data were generated by Quest Pharmaceutical Services in partnership with Agios Pharmaceuticals.
This study is part of a Cooperative Research and Development Agreement with Agios Pharmaceuticals, Inc, Cambridge, MA. This work was supported by the Division of Intramural Research of the National Heart, Lung, and Blood Institute and National Institute of Diabetes, and Digestive and Kidney Disease, National Institutes of Health.
Authorship
Contribution: S.L.T. is the principal investigator and supervised the study; J.Z.X., A.C., and S.L.T. conceptualized and designed the study; J.Z.X., A.C., I.F., R.P.C., and S.L.T. recruited and enrolled patients; A.C., I.F., R.P.C., D.L., and S.L.T. identified adverse events and determined their attributions; S.M.-M. provided clinical support; A.C., I.F., and S.L.T. wrote subsequent protocol amendments; K.L. and B.K. collected and processed blood samples; B.K., Q.L., and E.D. performed experiments to measure oxygen affinity (p50) and sickling kinetics (t50); C.H. provided oversight for the generation of pyruvate kinase, 2,3-diphosphoglycerate, and adenosine triphosphate (ATP) data; W.A.E. oversaw p50 and t50 data collection and provided input in the pharmacodynamics data analysis; N.A. and N.J. performed the statistical analysis; A.C., N.A., N.J., S.M.-M., and S.L.T. wrote and reviewed subsequent drafts that were reviewed by all authors; and all authors approved the final version of the manuscript for publication.
Conflict-of-interest disclosure: A.Y., M.W.-R., and C.H. are employees of, and shareholders in, Agios Pharmaceuticals, Inc. J.Z.X. reports research funding from GlaxoSmithKline (GSK) and served on an advisory committee for GSK and Agios Pharmaceuticals, Inc. The remaining authors declare no competing financial interests.
Correspondence: Swee Lay Thein, National Heart, Lung, and Blood Institute/National Institutes of Health, Building 10-CRC, Room 6S241, 10 Center Dr, Bethesda, MD 20892; email: sl.thein@nih.gov.
References
Author notes
Presented in abstract form at the 65th annual meeting of the American Society of Hematology, San Diego, CA, 9 December 2023.
Deidentified individual participant data that underlie the reported results will be made available 3 months after publication for a period of 5 years after the publication date from the corresponding author, Swee Lay Thein (sweelay.thein@nih.gov). Original data are available at https://doi.org/10.25444/nhlbi.29425343.
The full-text version of this article contains a data supplement.