Key Points
Donor variability is high regarding markers of physiologic stress and cannot be predicted by chronological storage age alone.
Patient serum iron and transfusion of PS-PE high red cell units were predictive of important clinical outcomes for adults with SCD.
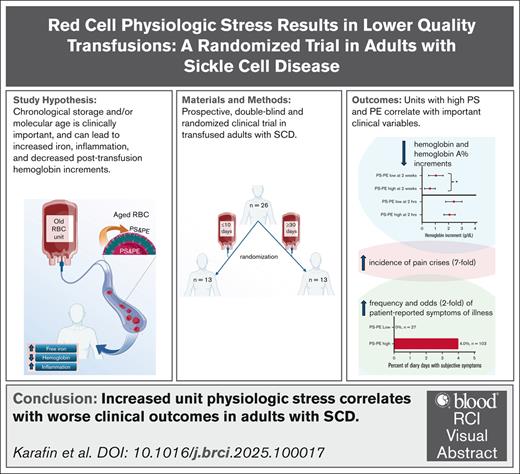
Visual Abstract
People with sickle cell disease (SCD) may be transfused with red cell units that are near the end of their storage life, exposing them to components of the red cell storage lesion. This study evaluated the clinical impact of storage age and red cell distress markers on chronically transfused adults with SCD. This randomized prospective clinical trial recruited 26 chronically transfused adult patients (aged >16 years) with SCD; 13 participants were randomized to each study arm, that is, targeted to receive only ≥30-day or ≤10-day stored red cell units for 3 consecutive outpatient transfusion events. The red cell units were evaluated via quantification of surface exposure of phosphatidylserine (PS) and phosphatidylethanolamine (PE). Differences in key clinical variables were also evaluated. We show that patients receiving units with higher surface-exposed PS and PE, regardless of storage age, had a reduced hemoglobin (Hb) increment at 2 weeks (PS-PE high, 0.59 g/dL; PS-PE low, 1.04 g/dL; P = .04), increased pain crises (incidence rate ratio, 7.44; 95% confidence interval [CI], 1.53-36.25), and higher odds of reported illness (odds ratio, 1.94; 95% CI, 1.06-3.54). Furthermore, posttransfusion serum iron predicted subsequent subjective symptoms of illness (receiver operating characteristic–area under the curve, 0.76) and correlated with smaller increases in HbA percent after transfusion (r =–0.36; P = .04). These data suggest that the physiologic state of transfused red cells may deleteriously affect patient outcomes. Future studies focusing on identifying and then avoiding lower-quality red cell units in high-risk patients with SCD could enhance patient safety. This trial was registered at www.clinicaltrials.gov as #NCT03704922.
Introduction
Red cell transfusions are critically important for patients with sickle cell disease (SCD). For example, red blood cell (RBC) transfusions correct anemia, decrease sickle hemoglobin (HbS) percentage, suppress endogenous RBC synthesis, and reduce hemolysis.1 When administered chronically, typically monthly, these transfusions can successfully mitigate severe anemia and risks of stroke, acute chest syndrome, and multiorgan failure.2-4 Despite the advent of innovative curative therapies, RBC transfusion continues to be used in this population, inspired by well-established and evolving indications for chronic transfusion therapy.2-4
RBC units in the United States can be stored for up to 42 days in additive solution, but this refrigerated storage comes at the cost of the progressive accumulation of biochemical, metabolic, and morphologic changes, collectively referred to as the RBC storage lesion.5 Extended storage time alters RBC membrane permeability, eventually inducing morphologic changes, decreased deformability, irreversible sphericity, and a diminished ability to traverse the microcirculation after transfusion.6 Although these processes are observed during storage of all RBCs, their onset, progression, and severity vary from unit to unit as a function of donor-dependent and processing factors.7-29
The impact of the storage lesion on adults with SCD has not been studied extensively. Prior randomized trials (eg, ABLE, ARIPI, RECESS, INFORM), which excluded people with SCD, found no relationship between RBC storage age and adverse outcomes.30-33 To our knowledge, the only prospective trial, conducted in severely anemic African children with malaria (n = 251) and SCD (n = 39), found no difference in the ability of older (stored 25-35 days) vs younger units (stored 1-10 days) to correct lactate levels.34 However, this study did not address a key question: whether transfusions of older stored RBCs lead to an increased risk of adverse outcomes in people with SCD.
Unlike the other studied populations, patients with SCD are unique. Morbidity in SCD involves complex interactions between white blood cells (WBCs), platelets, endothelial cells, as well as the sickled RBCs themselves.35 Phosphatidylserine (PS) and phosphatidylethanolamine (PE) exposure on donor RBCs increases with storage,36-39 and both are associated with increases in microparticle concentration and release of cell-free Hb39; PS exposure on transfused RBCs, uniquely, increases further when exposed to the plasma of patients with SCD ex vivo, especially from patients who are experiencing a vaso-occlusive crisis.40 PS exposure on RBCs may also promote vaso-occlusion through changes to the immune system, increased adherence to the vascular endothelium, complement activation, and thrombin generation.41-48
PS and PE receptors on phagocytes both contribute to extravascular phagocytosis of PS- and PE-exposing RBCs.47 In the context of transfusion, the clearance of RBCs exposing high levels of PS and PE could contribute to the release of excess iron, which can generate reactive oxygen species, promote inflammation, and enhance the proliferation of ferrophilic microorganisms.49-57 Thus, transfusions of units with high-surface PS-PE could potentially promote tissue damage and increase infection risk; an association that we found in a modest-sized retrospective study.58
Despite concerns, there continues to be clinical equipoise regarding the safety of using chronologically older units for patients with SCD.59-61 This study directly addresses this significant knowledge gap by focusing on a controlled cohort of adult patients with SCD, prospectively randomized to receive 3 consecutive transfusion events exclusively with either fresher (≤10 days) or longer-stored RBCs (≥30 days). The clinical and laboratory outcomes of these transfusion recipients were evaluated as a function of both chronological storage and molecular age (ie, surface levels of PS and PE). The primary aim of this trial was to determine whether chronological storage and/or molecular age were associated with any differences in clinical or laboratory outcomes in this unique patient cohort.
Methods
Study design
The protocol for this randomized clinical trial and its associated primary and secondary study aims were previously described in detail.62 In brief, patients were randomized to receive RBCs stored either ≤10 days or ≥30 days at 3 consecutive outpatient transfusion events. The assigned study arm was known only to the blood bank staff. Each of the 3 participating institutions had their own transfusion protocol, but in general, 2 units were used with each event, with fewer or greater units being used depending on the presenting Hb level. The critical inclusion criteria included the following: (1) patient age between 16 and 60 years; (2) confirmed SCD by Hb analysis; (3) ongoing chronic RBC transfusion therapy; and (4) outpatient status at the time of all study transfusions. Patients were not eligible for this study if they (1) received manual or automated exchange transfusions; (2) required crossmatch-incompatible RBCs; and/or (3) were participating in another therapeutic trial.
Laboratory measures and flow cytometry analyses
PE and PS surface positivity were measured by flow cytometry using unit links/segments. The method used has been described previously.39 PS-PE–high units were defined as units in which the percent of total analyzed RBCs for surface PE and/or PS at the time of testing exceeded 8% and/or 3%, respectively; these were selected a priori as the points at which the sigmoidal curves of PS and PE increasing over storage time entered their log phase, which was determined to be the mean value of PS and PE noted at day 21.39 This cutoff was also determined to be biologically meaningful, because units stored beyond day 21 had a more rapid increase in donor RBC adhesion to endothelial cells.63 Therefore, each transfusion event was defined as either being PS-PE low (ie, all units had PE and/or PS values <8% and/or <3%, respectively), PS-PE mixed (where one RBC unit was young and 1 was old), or PS-PE high (where all units had PE and/or PS values ≥8% and/or ≥3%, respectively). The gating strategy for surface PS and PE determination is shown in supplemental Figure 1.
Whole blood samples were obtained from each patient before transfusion and at 2 hours, 24 hours, and 14 days after transfusion.62 Clinical laboratory measurements obtained for each patient included complete blood count, Hb electrophoresis, haptoglobin, routine chemistry parameters, serum iron and transferrin saturation, lactate dehydrogenase, urinalysis, and blood culture.62
Daily diary
Each patient was provided with a diary (supplemental Figure 2). Each day, patients rated their pain on an ordinal numeric pain scale (0-10), indicating whether the pain was consistent with a “crisis,” whether they used a health care facility, whether they felt they had an infection, and the type and dose of opioids used for pain control. Subjective symptoms of infection were also documented along with the type, duration, and dose of any antibiotics taken. Emergency department visits, pain clinic visits, hospitalization events, and the reasons for them were recorded from the electronic medical record on patient adverse event (AE) forms.
Statistical analysis
All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC). Laboratory values and clinical outcomes were compared for participants by study arm using an intent-to-treat analysis. As a sensitivity analysis, results were also compared using a per-protocol analysis, that is, limiting the sample to those transfusion visits that adhered to the protocol-specified allocation. Additionally, we compared laboratory values and clinical outcomes for PS-PE–low, PS-PE–mixed, and PS-PE–high transfusion events. Posttransfusion laboratory values were compared between groups using linear mixed models, to account for repeat transfusion visits (up to 3) per participant and repeated posttransfusion laboratory assessments (2 hours, 24 hours, and 2 weeks after transfusion) after each transfusion.
Separate models were constructed for each laboratory analyte, adjusting for pretransfusion values, the number of RBC units transfused with each visit, and posttransfusion measurement time points. The 2 key laboratory outcome variables of interest included changes from before transfusion to 2 hours, 24 hours, 2 weeks, and before the next outpatient transfusion in (1) Hb (grams per deciliter) and (2) HbA, both as a percentage and g/dL. We further evaluated HbA decrement as a rate (percent per day and g/dL per day). Repeated posttransfusion laboratory assessments were analyzed by treating participant-specific transfusion visits as statistical clusters, implementing an autoregressive covariance structure to account for the greater similarity in laboratory results assessed more proximally to each other than those distanced by greater amounts of posttransfusion time. To assess potential modification of associations by laboratory measurement time point (eg, differing slopes or rate of posttransfusion change in analyte among the compared groups), the multiplicative interaction between RBC unit age and laboratory time point was tested in the models.
Pain scores assessed at transfusion visits 2 and 3, as well as the pain diary, were also analyzed using linear mixed models, treating study participants as statistical clusters and accounting for the baseline pain score at transfusion visit 1, the number of RBC units transfused, and the transfusion event number. Physician-documented and self-reported infection-like symptoms were compared between recipients of fresh vs longer-stored RBCs and among PS-PE levels, using logistic regression models with generalized estimator equations to account for repeated symptom assessments. Only patients who specifically reported that they believed that they had an infection were included in this analysis (supplemental Figure 2, question 5).
The study was conducted in accordance with the Declaration of Helsinki and good clinical practice guidelines. The Medical College of Wisconsin, University of North Carolina at Chapel Hill, and Emory University review boards approved the protocol independently, and there was no central institutional review board. All research participants provided written informed consent before study participation.
Results
Patent population
The characteristics of the consented patients are summarized in Table 1 and supplemental Table 1. Twenty-six patients were randomized and received at least 1 study transfusion, with 13 patients randomized to the ≤10-day arm and 13 randomized to the ≥30-day arm. Overall, there were 68 randomized transfusion events; 13 of those events (19.1%) did not meet the study storage age requirements. Most patients received transfusion with 2 RBC units per event (60/68 [88.2%]). Demographically, patients randomized to each arm were similar in most respects; however, there were more females in the ≥30-day arm (77% vs 46.2%). Importantly, no patient received their first study transfusion within a month of their last hospitalization due to a pain crisis, and no patients started hydroxyurea, voxelotor, l-Glutamine, or crizanlizumab during the study period.
Unit characterization
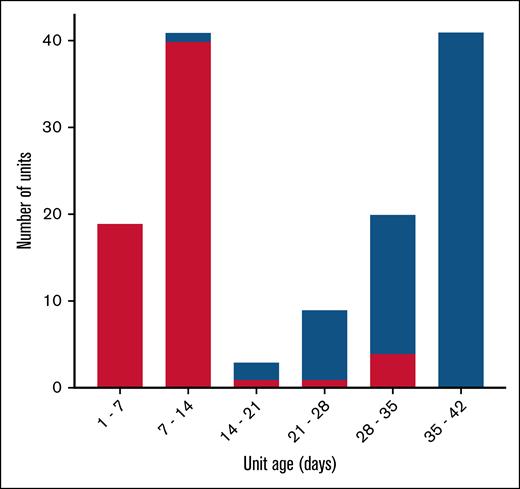
Over the study period, 132 RBC units were transfused: 65 units (49.2%) to patients in the ≤10-day arm and 67 (50.8%) to those in the ≥30-day arm. There was very little overlap in the storage ages of transfused units (Figure 1), with 8 transfused units (12.3%) stored for >10 days in the ≤10-day arm and 11 transfused units (16.4%) stored for <30 days in the ≥30-day arm (P = .6). The average unit age in the ≤10-day arm was 9.2 (±6.7) days, and the average unit age in the ≥30-day arm was 34.1 (±6.6) days. Most transfused units were stored in Adsol preservative-1 (87.9%), with a few stored in Adsol preservative-3 (10.6%) or citrate-phosphate-adenine anticoagulant preservative (1.5%). There was no significant difference between the study arms regarding the type of additive solution used (P = .4).
Histogram of storage age of the units transfused by study arm (red, ≤10-day arm; blue, ≥30-day arm). There were 8 transfused units (12.3%) stored for >10 days in the fresh unit arm, and 11 transfused units (16.4%) stored for <30 days in the old unit arm (P = .6).
Histogram of storage age of the units transfused by study arm (red, ≤10-day arm; blue, ≥30-day arm). There were 8 transfused units (12.3%) stored for >10 days in the fresh unit arm, and 11 transfused units (16.4%) stored for <30 days in the old unit arm (P = .6).
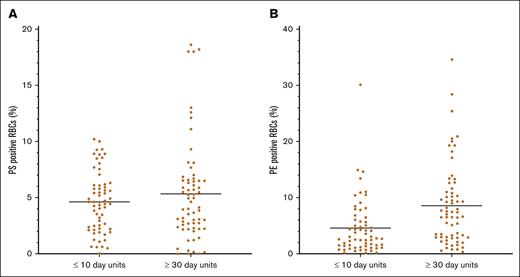
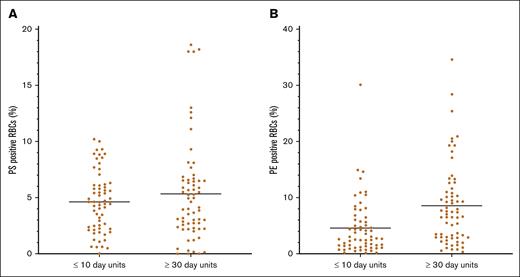
The mean surface PS expression was slightly higher in RBCs stored ≥30 days (4.6% ± 2.7% in the ≤10-day arm; 5.3% ± 4.5% in the ≥30-day arm; P = .3). However, units transfused in the ≥30-day arm had significantly higher numbers of surface PE expression–positive RBCs than those in the ≤10-day arm (4.6% ± 5.0% in the ≤10-day arm and 8.5% ± 7.2% in the ≥30-day arm; P = .0004; Figure 2). This relationship did not change when the units that did not meet the required storage age were excluded.
Distribution of surface-exposed PS (A) and PE (B) on stored RBCs. Aliquots were evaluated by flow cytometry. There was no significant difference between the 2 arms regarding PS surface positivity, but units transfused in the ≥30-day arm had a significantly higher percent of PE-positive RBCs than those in the ≤10-day arm (P = .0004).
Distribution of surface-exposed PS (A) and PE (B) on stored RBCs. Aliquots were evaluated by flow cytometry. There was no significant difference between the 2 arms regarding PS surface positivity, but units transfused in the ≥30-day arm had a significantly higher percent of PE-positive RBCs than those in the ≤10-day arm (P = .0004).
Eighteen transfused units in the ≤10-day arm were defined as PS-PE low (18/63 [28.6%]; per protocol, 16/57 [28%]; P = 1.0). In contrast, 58 (58/67 [86.6%]; per protocol, 48/56 [85.7%]; P = 1.0) of the transfused RBC units in the ≥ 30-day arm were PS-PE high. Overall, there were significantly more PS-PE low RBC units transfused to patients in the ≤10-day arm than in the ≥30-day arm (P = .03). There were no statistically significant differences regarding patient demographic characteristics among those who received PS-PE low, PS-PE mixed, or PS-PE high transfusion events (supplemental Table 2).
Impact of storage and PS-PE expression levels related to changes in clinical laboratory values
Unadjusted mean (±standard deviation) laboratory values for all evaluated posttransfusion time points revealed that RBC transfusion events in the ≥30-day arm were associated with higher recipient WBC count (P = .04), serum iron (P = .0004), serum ferritin (P = .04), unsaturated iron binding capacity (P = .002), and transferrin saturation (P = .001; supplemental Figure 3). No significant differences were identified for Hb, HbA percent, or HbA (g/dL) by study arm; however, quantitatively greater decreases in Hb and HbA percent were observed between transfusion events for those in the ≥30-day arm.
Linear mixed models of the laboratory data revealed that RBC transfusion events in the ≥30-day arm were significantly associated with higher potassium (P = .007), serum iron (P < .0001), and transferrin saturation (P = .006), as well as lower bicarbonate (P = .002) and percent monocytes (P = .02). Again, there were no statistically significant overall differences between the study arms for Hb, hematocrit, HbA percent, or HbA (g/dL; Table 2).
There were no statistically significant group differences in HbA percent per day, HbA (g/dL per day), or Hb increment by the assigned storage arm. However, those receiving ≥30-day units had quantitatively greater drops in HbA percent per day during the first 2 weeks after transfusion (Figure 3).
Adjusted change in Hb (with 95% CI) and HbA percent per day from the pretransfusion value across all 3 transfusion events, stratified by phlebotomy time point and adjusted for the number of units transfused. (A-B) Hb increment 2 hours after transfusion (34 pre-to-post deltas based on 14 unique participants), 24 hours after transfusion (29 pre-to-post deltas based on 14 unique participants), and 2 weeks after transfusion (55 pre-to-post deltas based on 24 unique participants) are shown by study arm (A) and by PS-PE surface content (B). No significant differences were noted by study arm at 2 hours or 2 weeks after transfusion but was significant at 24 hours after transfusion (2.22 vs 3.23 g/dL; P = .007). Transfusion episodes classified as PS-PE high had significantly smaller Hb increments at both 24 hours and 2 weeks after transfusion, compared to PS-PE mixed and low transfusion events (24 hours, 2.38 vs 2.82 vs 2.89 g/dL [P = .02]; 2 weeks, 0.59 vs 0.89. vs 1.04 g/dL [P = .04]). (C-D) The adjusted mean decrement in HbA percent per day (with 95% CI) across all 3 transfusion events during the first 2 weeks after transfusion, the second 2 weeks after transfusion, and overall (change between exchanges) are shown by study arm (C) and during the first 2 weeks and second 2 weeks post-transfusion by PS-PE surface content (D). Transfusion-specific decrement per day from 2 hours to 2 weeks after transfusion is based on 28 transfusions involving 13 unique patients. Transfusion-specific decrement per day from 2 weeks to before the next transfusion included 19 total assessments from 11 unique patients. The overall change in HbA percent per day by study arm was –0.24% for those receiving ≤10-day units and –0.14% for those receiving ≥30-day units (P = .3). There were no significant differences in the change in HbA percent per day by study arm or PS-PE, but PS-PE high and ≥30-day units had greater decrements in the first 2 weeks.
Adjusted change in Hb (with 95% CI) and HbA percent per day from the pretransfusion value across all 3 transfusion events, stratified by phlebotomy time point and adjusted for the number of units transfused. (A-B) Hb increment 2 hours after transfusion (34 pre-to-post deltas based on 14 unique participants), 24 hours after transfusion (29 pre-to-post deltas based on 14 unique participants), and 2 weeks after transfusion (55 pre-to-post deltas based on 24 unique participants) are shown by study arm (A) and by PS-PE surface content (B). No significant differences were noted by study arm at 2 hours or 2 weeks after transfusion but was significant at 24 hours after transfusion (2.22 vs 3.23 g/dL; P = .007). Transfusion episodes classified as PS-PE high had significantly smaller Hb increments at both 24 hours and 2 weeks after transfusion, compared to PS-PE mixed and low transfusion events (24 hours, 2.38 vs 2.82 vs 2.89 g/dL [P = .02]; 2 weeks, 0.59 vs 0.89. vs 1.04 g/dL [P = .04]). (C-D) The adjusted mean decrement in HbA percent per day (with 95% CI) across all 3 transfusion events during the first 2 weeks after transfusion, the second 2 weeks after transfusion, and overall (change between exchanges) are shown by study arm (C) and during the first 2 weeks and second 2 weeks post-transfusion by PS-PE surface content (D). Transfusion-specific decrement per day from 2 hours to 2 weeks after transfusion is based on 28 transfusions involving 13 unique patients. Transfusion-specific decrement per day from 2 weeks to before the next transfusion included 19 total assessments from 11 unique patients. The overall change in HbA percent per day by study arm was –0.24% for those receiving ≤10-day units and –0.14% for those receiving ≥30-day units (P = .3). There were no significant differences in the change in HbA percent per day by study arm or PS-PE, but PS-PE high and ≥30-day units had greater decrements in the first 2 weeks.
When evaluating the Hb increments by surface PS and PE expression, those transfusion events that were defined as PS-PE low yielded a significantly higher Hb increment, compared to pretransfusion levels, than transfusion events with PS-PE–high units, at both 24 hours (PS-PE high, 2.38 g/dL; PS-PE mixed, 2.82 g/dL; PS-PE low, 2.89 g/dL; P = .02) and 2 weeks after transfusion (PS-PE high, 0.59 g/dL; PS-PE mixed, 0.89 g/dL; PS-PE low, 1.04 g/dL; P = .04; Figure 3). However, there were no statistically significant group differences in HbA percent per day or HbA (g/dL) per day with regard to the RBC unit PS-PE level. Similar to the ≥30 day units, PS-PE–high units had quantitatively greater drops in HbA percent per day during the first 2 weeks after transfusion (Figure 3).
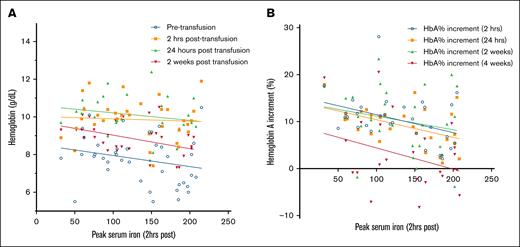
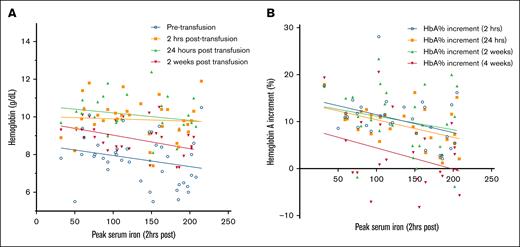
In contrast, the peak serum iron at 2 hours after transfusion was significantly negatively correlated with a reduced HbA percent increment at 2 hours (r = –0.36; P = .04) and 24 hours after transfusion (r = –0.39; P = .03). Peak serum iron at 2 hours after transfusion was also significantly negatively correlated with Hb levels at 2 weeks after transfusion (r = –0.42; P = .01; Figure 4).
Scatterplot demonstrating the negative correlation (best fit regression lines are shown) between the peak serum iron at 2 hours after transfusion and the decrease in Hb (A) and HbA percent decrement after transfusion (B). Although the negative correlation was seen at all time points observed, the negative correlation was significant for Hb at 2 weeks after transfusion (n = 32; r = –0.42; P = .01) and HbA percent increment at 2 hours (n = 32; r = –0.4; P = .03) and 24 hours (n = 28; r = –0.5; P = .01) after transfusion.
Scatterplot demonstrating the negative correlation (best fit regression lines are shown) between the peak serum iron at 2 hours after transfusion and the decrease in Hb (A) and HbA percent decrement after transfusion (B). Although the negative correlation was seen at all time points observed, the negative correlation was significant for Hb at 2 weeks after transfusion (n = 32; r = –0.42; P = .01) and HbA percent increment at 2 hours (n = 32; r = –0.4; P = .03) and 24 hours (n = 28; r = –0.5; P = .01) after transfusion.
Relationships between RBC unit storage age, PS-PE levels, and infection
Blood and urine cultures were obtained before each study transfusion event. Two urine cultures were positive after the first randomized transfusion: one for Escherichia coli and one without further speciation; both were positive after transfusion of PS-PE–high units (one in a patient randomized to the ≤10-day arm and one randomized to the ≥30-day arm). Additionally, 1 patient was hospitalized for 9 days due to documented sepsis from Haemophilus influenzae ∼3 weeks after the transfusion of 2 PS-PE–high units (≥30-day arm). Lastly, 1 individual had documented influenza ∼3 weeks after the transfusion of 2 PS-PE–high units (≤10-day arm).
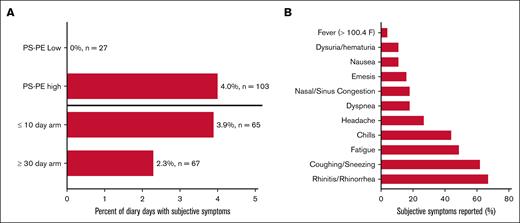
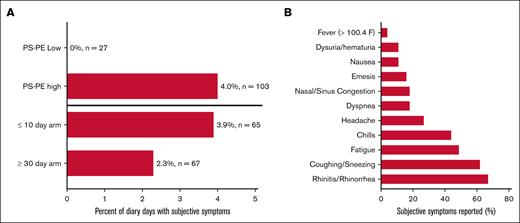
Patients were asked to comment on whether they felt that they had an infection and to describe their symptoms both by diary and during pretransfusion physical examination. Seven patients (27%) reported subjective symptoms of illness over 45 cumulative diary days. Forty of those 45 days (88%) occurred after transfusion with PS-PE–high RBC units (4.0% of diary days; P = .004), and no symptoms were noted in those who received PS-PE–low units. The most common symptoms noted were suggestive of an upper respiratory illness (ie, cough, stuffy or runny nose, and sinus pain; Figure 5). Similarly, 7 patients (27%) reported symptoms of subjective illness during 14 of their pretransfusion physical examinations (20.8%) after the first study transfusion. Again, receiving only PS-PE–high RBCs was associated with higher odds of reporting symptoms, with 11 of those putative symptoms (78.6%) occurring after receiving only PS-PE–high units (odds ratio, 1.94; 95% confidence interval [CI], 1.06-3.54).
Patient-reported symptoms of illness during the study interval. Seven of the 26 patients reported at least 1 subjective symptom of possible illness during the study interval, accounting for 45 of the 1434 diary days (3.1%) recorded (A). Forty of those 45 days (88%) were after transfusion with PS-PE high units (4.0% of diary days; 103 involved transfused units; P = .004), and no symptoms were noted in those who received PS-PE–low units (0% of diary days and 27 involved transfused units). The odds of having subjective symptoms reported during the physical examination after transfusion with PS-PE–high units was 1.94 (95% CI, 1.06-3.54). No statistically significant differences were observed when comparing study arms (P =.1; odds ratio, 1.41; 95% CI, 0.25-7.87). (B) The most common symptoms noted were upper respiratory in nature (ie, rhinitis, cough, and fatigue).
Patient-reported symptoms of illness during the study interval. Seven of the 26 patients reported at least 1 subjective symptom of possible illness during the study interval, accounting for 45 of the 1434 diary days (3.1%) recorded (A). Forty of those 45 days (88%) were after transfusion with PS-PE high units (4.0% of diary days; 103 involved transfused units; P = .004), and no symptoms were noted in those who received PS-PE–low units (0% of diary days and 27 involved transfused units). The odds of having subjective symptoms reported during the physical examination after transfusion with PS-PE–high units was 1.94 (95% CI, 1.06-3.54). No statistically significant differences were observed when comparing study arms (P =.1; odds ratio, 1.41; 95% CI, 0.25-7.87). (B) The most common symptoms noted were upper respiratory in nature (ie, rhinitis, cough, and fatigue).
To ensure that the a priori PS and PE cutoffs were optimized, we evaluated a series of receiver operator curves (supplemental Figure 4). Most of the predictive capability for subjective symptoms of illness was driven by PE (PE, area under the curve [AUC] = 0.60; PS, AUC = 0.48; model incorporating both PS-PE, AUC = 0.61). Lastly, we evaluated whether serum iron correlated with these subjective reports of illness and found that the peak serum iron at 2 hours after transfusion was also highly predictive (AUC = 0.76).
Hospitalizations, AEs, and pain crises
Seven patients (27%) had a total of 14 severe AEs (SAEs) resulting in hospitalization. Nine hospitalizations (64%) were for complicated pain crises, 2 (14%) for hyperkalemia, 1 (7.1%) for hyperglycemia, 1 (7.1%) for sepsis, and 1 (7.1%) for fistula complications. Although 8 SAEs (57%) occurred in patients randomized to the ≤10-day arm, most occurred after transfusion of at least 1 PS-PE–high RBC unit (12/14 events [86%]). Nonetheless, there was no overall statistically significant relationship between SAEs and study group, metabolic age, or serum iron (by study arm, P = .7; by PS-PE, P = .9; by serum iron, P = .4).
A total of 28 nonsevere AEs were recorded for 8 patients (31%) during the study period. These events required either a visit to the emergency department or a pain clinic. Most of the AEs reported were pain crises (24/28 [85.7%]), followed by 3 episodes of priapism (10.7%) and 1 episode of influenza (3.6%). Most of these events were in patients randomized to the ≥30-day arm (5/13 patients [38.5%]; 25/28 events [89.3%]), and all of these events occurred after transfusion of at least 1 PS-PE–high unit (28/28 [100%]).
The overall adjusted incidence rate ratio of having a pain event was significantly higher in those randomized to the ≥30-day arm (8.18; 95% CI, 2.04-32.86; P = .003) and in those receiving PS-PE–high units (7.44; 95% CI, 1.53-36.25; P = .01). Most of the predictive capability for pain crises was driven by PS (PE, AUC = 0.59; PS, AUC = 0.64; model incorporating both PS-PE, AUC = 0.68). Peak serum iron at 2 hours after transfusion was not associated with the risk of pain crises (P = .8).
Across the entire cohort, the mean daily pain score was 2.9 (standard deviation, 3.2; range, 0-10). There was no significant changes in daily pain over time by randomized patient group, PS-PE levels (study arm, P = .26; molecular age, P = .46; supplemental Figure 5), or posttransfusion peak serum iron levels (r = –0.06; P = .8).
Discussion
To our knowledge, this prospective randomized clinical trial is the first to evaluate the direct clinical impact of donor RBC storage age and surface PS and PE expression (ie, molecular age) on chronically transfused patients with SCD. We found that RBC units transfused after ≥30 days of storage were associated with increased recipient serum iron, which, in turn, was associated with increased subjective symptoms consistent with illness and decreased Hb and HbA percent increments. RBC units transfused after ≥30 days of storage was also associated with an eightfold increased incidence of documented pain crises. Increased RBC surface exposure of PS and PE before transfusion, independent of storage age, was associated with a more rapid fall in Hb levels after transfusion, objective documentation of infections, a sevenfold increased incidence of pain crises, and a twofold increased odds for subjective symptoms concerning for illness.
A novel finding from this randomized trial is that some clinical outcomes could not be predicted by unit storage age alone (ie, the randomized storage arm); instead, RBC surface exposure of PS and PE and peak serum iron after transfusion proved to be important predictors of clinical outcomes. The “iron hypothesis” suggests that transfusion of longer-stored RBC units leads to greater increases in serum iron, which was associated with subsequent AEs, including infection.5,49,53 This randomized clinical trial demonstrates that iron was, indeed, increased in those randomized to the longer-stored unit arm, but the peak concentration of serum iron levels varied (∼30% of the highest serum iron levels recorded at 2 hours after transfusion were from patients randomized to the ≤10-day arm). The reasons for this are unclear but may be due to either recipient-specific factors, such as preexisting iron overload, or the recipient’s basal hemolytic rate, which may explain the serum iron level’s association with Hb and HbA percent over time. Nonetheless, the findings generally support the established association between the increased availability of iron in circulation and adverse patient outcomes, such as infection.55
The clinical associations with RBC surface-exposed PS and PE in this trial represent direct evidence that supports the growing understanding that unit-specific factors are critically important for predicting transfusion efficacy.64,65 Despite restricting RBC transfusions in this trial to units stored for ≤10 days and ≥30 days, we still identified a wide variation in surface PS and PE expression. There are many possible explanations for this variability. For example, recent studies have identified that blood donor sex, Rh status, and smoking affect Hb increments after RBC transfusion. Moreover, donor-specific genetic polymorphisms, including, but not limited to, the G6PD, SEC14L4, HBA2, MYO9B, and STEAP3 genes are also associated with unit efficacy after transfusion (ie, increased recipient transfusion requirements in the subsequent 48 hours).65,66 Although we did not evaluate donor unit–specific characteristics in this trial, our findings support the concept that precision medicine approaches to transfusion may be important for improving transfusion outcomes in chronically transfused individuals.
Pain crises are the most common morbidity for adults with SCD.1 We identified that 27 reported AEs were related to pain. All of these events occurred after transfusion of PS-PE–high units. The relationship between surface PS exposure on RBCs and vaso-occlusion is well-known. We identified more than sevenfold increased odds of acute pain crises from PS-PE–high RBCs and units in the ≥30-day arm (nearly 90% of which were PS-PE high). This finding supports the known literature that a large bolus of PS-high RBCs, generally associated with older units, could trigger an increase in the risk of acute vaso-occlusive pain events.42-48 To our knowledge, however, no studies have yet addressed whether surface PE has similar effects on patient physiology. These observations would also need confirmation in future studies.
Infections are clinically relevant complications for patients with SCD, who are at higher risk for both viral and bacterial infections even without transfusion therapy.67-73 In this trial, we found a potential association between infection and the transfusion of PS-PE–high units. This association is intriguing, because there are known ligands for PS and PE, such as CD300a, that are correlated with infection risk.74-76 However, this association should be interpreted with caution, because more studies would be needed to draw a definitive causal relationship. Specifically, we did not identify any significant decrease in C-reactive protein, WBC, or any specific immune cell type to suggest that a loss of immunity occurred in those receiving PS-PE–high RBCs. Moreover, we identified that serum iron was also highly correlated with infection symptoms, which could implicate a role for nutritional immunity.5,49,53 Nonetheless, given the very small number of documented infections in this study, larger longitudinal and prospective studies will be needed.
Although the results from this small trial do not definitively prove that surface-exposed PS and PE or serum iron as the best or only markers for predicting donor RBC safety and efficacy in patients with SCD, they do strongly support the concept that better predictors of transfusion efficacy are needed. To this end, many novel methods are now being evaluated, but none, to our knowledge, are currently ready for real-time clinical decision-making; thus, additional randomized clinical trials, complemented by large-scale observational studies, are needed to determine which, if any, of these techniques will be practical and also provide the best clinical predictive value.77
This study has several limitations. First, the trial did not reach the planned recruitment goal of 40 randomized patients, predominantly due to both changes in general practice prioritizing exchange transfusions over simple transfusions and the disruption in recruitment because of the COVID-19 pandemic. Consequently, the lack of statistical significance observed for some variables may been due to a lack of power. However, it is notable that statistically significant differences by study arm, surface PS-PE, and serum iron in this relatively small cohort could be identified. Second, the study design, although randomized, was not ideal. For example, study patients and coordinators could inadvertently be unblinded because the unit labels were not altered. Nonetheless, it is unlikely that the main study aims (PS and PE exposure and clinical laboratory measures) would be affected by any unintentional unblinding. Furthermore, we did not directly account for RBC:platelet aggregates during the study; thus, PS- and PE-positive platelets could have played a role in the results reported. However, subsequent control experiments performed revealed that <0.2% of the events reported in both older and fresher RBC units represented aggregates; therefore, the impact of residual platelets in affecting the overall results is likely minor (supplemental Figure 6). Third, the subjective symptoms of infection reported by diary and physical examination could not be proven as representing a true infection. Nonetheless, we did objectively identify 4 infections, all of which occurred after transfusion with PS-PE–high units. Fifth, most of the recruited cohort (77%) was iron overloaded. Given that many patients now receive automated exchanges and chelation therapy to minimize iron overload, our findings may be less representative of the current population of chronically transfused patients with SCD. Lastly, many established storage quality variables could not be evaluated or controlled for in this clinical trial.
This prospective, randomized clinical trial of 26 chronically transfused patients with SCD revealed that increased chronological storage age correlated with both increased surface PS and PE and increased serum iron levels. However, donor unit variability was high. In addition, independent of study arm, the surface exposure of PS and PE on transfused RBCs and posttransfusion serum iron levels correlated with important clinical outcomes, including posttransfusion Hb increment, posttransfusion HbA percent, documented pain crises, and possible infections. Consequently, our findings do not yet definitively suggest that current general practices should be altered. Nonetheless, to maximize patient safety in this cohort, future sufficiently powered studies will need to evaluate the role of markers of donor RBC unit quality in minimizing posttransfusion complications.
Acknowledgments
The authors thank Angelo D’Alessandro and Steven Spitalnik for their critical review and comments on this manuscript. They thank Pippa Simpson who helped with the original statistical plan and randomization scheme for this clinical trial. They acknowledge the Versiti Blood Research Institute Shared Resources Core Facility (Research Resource Identifier SCR_025503), supported by the Versiti Blood Research Institute Foundation, for its services and research coordinator support.
This study was supported by the National Heart, Lung, and Blood Institute, National Institutes of Health (K23HL136787 and R01HL148151; M.S.K.).
Authorship
Contribution: M.S.K. drafted the first version of the manuscript; M.S.K., J.J.F., and J.A.L. participated in the study design; R.M.F., M.S.K., J.A.L., and J.J.F. helped implement the study at all sites; R.J., S.Z.-J., S.M.J., H.E.B., A.C., and D.W. helped recruit and complete the study protocol; M.C.C., S.R.W., O.K., and A.I. helped with data analysis; A.I. developed the visual abstract; and all authors reviewed and edited the manuscript.
Conflict-of-interest disclosure: M.S.K. works as a paid consultant for Westat, Inc. R.M.F. serves on a medical advisory board for Pfizer and Cerus; has received research funding from Cerus; and serves as a consultant for REDS-IV-P, which was funded by the National Institutes of Health/National Heart, Lung, and Blood Institute. J.J.F. received honoraria from Bayer; and research funding from Forma Therapeutics, Shire/Takeda, and Rigel. J.A.L. receives research support from Pfizer, Novo Nordisk, and the American Society of Hematology. The remaining authors declare no competing financial interests.
Correspondence: Matthew S. Karafin, Pathology and Laboratory Medicine, University of North Carolina School of Medicine, 101 Manning Dr, Chapel Hill, NC 27514; email: matthew.karafin@unc.edu.
References
Author notes
Original data are available from the corresponding author, Matthew S. Karafin (matthew.karafin@unc.edu), on request.
The full-text version of this article contains a data supplement.

![Adjusted change in Hb (with 95% CI) and HbA percent per day from the pretransfusion value across all 3 transfusion events, stratified by phlebotomy time point and adjusted for the number of units transfused. (A-B) Hb increment 2 hours after transfusion (34 pre-to-post deltas based on 14 unique participants), 24 hours after transfusion (29 pre-to-post deltas based on 14 unique participants), and 2 weeks after transfusion (55 pre-to-post deltas based on 24 unique participants) are shown by study arm (A) and by PS-PE surface content (B). No significant differences were noted by study arm at 2 hours or 2 weeks after transfusion but was significant at 24 hours after transfusion (2.22 vs 3.23 g/dL; P = .007). Transfusion episodes classified as PS-PE high had significantly smaller Hb increments at both 24 hours and 2 weeks after transfusion, compared to PS-PE mixed and low transfusion events (24 hours, 2.38 vs 2.82 vs 2.89 g/dL [P = .02]; 2 weeks, 0.59 vs 0.89. vs 1.04 g/dL [P = .04]). (C-D) The adjusted mean decrement in HbA percent per day (with 95% CI) across all 3 transfusion events during the first 2 weeks after transfusion, the second 2 weeks after transfusion, and overall (change between exchanges) are shown by study arm (C) and during the first 2 weeks and second 2 weeks post-transfusion by PS-PE surface content (D). Transfusion-specific decrement per day from 2 hours to 2 weeks after transfusion is based on 28 transfusions involving 13 unique patients. Transfusion-specific decrement per day from 2 weeks to before the next transfusion included 19 total assessments from 11 unique patients. The overall change in HbA percent per day by study arm was –0.24% for those receiving ≤10-day units and –0.14% for those receiving ≥30-day units (P = .3). There were no significant differences in the change in HbA percent per day by study arm or PS-PE, but PS-PE high and ≥30-day units had greater decrements in the first 2 weeks.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodrci/1/3/10.1016_j.brci.2025.100017/2/m_brci_rci-2025-000145-gr3.jpeg?Expires=1769229384&Signature=cXWiSF78A~xtKy1QDuyU887ZCX4PU8uzRX96xyU2LPOvup8y9unk5M7HnhcHv691J5s4tDMUD5U7VBhUWYaZGpJlw2GrOb7RkTC~ufHLvVOioycKsOIXPu-HHndyzswKMmen5Qzkcs~5Cr8QnHLTV7JQoxAUf7xW~BqicmxcfqJMe3ncEzcUs1sTRQxpDcuOBzpOaybVMDkhzxjBaRc69x0HwDdY3iKmbksbAUMNEfgbMnx80sKejv4dGpGdtNqywDVwZIBzqE8Mq75NYDTOKFHZy97OpXGqeCsW1V2h-0A8ZEn2oewMLx~Bjyybr3pumMyCh5uZEkqWo6bgTKAOGg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)



![Adjusted change in Hb (with 95% CI) and HbA percent per day from the pretransfusion value across all 3 transfusion events, stratified by phlebotomy time point and adjusted for the number of units transfused. (A-B) Hb increment 2 hours after transfusion (34 pre-to-post deltas based on 14 unique participants), 24 hours after transfusion (29 pre-to-post deltas based on 14 unique participants), and 2 weeks after transfusion (55 pre-to-post deltas based on 24 unique participants) are shown by study arm (A) and by PS-PE surface content (B). No significant differences were noted by study arm at 2 hours or 2 weeks after transfusion but was significant at 24 hours after transfusion (2.22 vs 3.23 g/dL; P = .007). Transfusion episodes classified as PS-PE high had significantly smaller Hb increments at both 24 hours and 2 weeks after transfusion, compared to PS-PE mixed and low transfusion events (24 hours, 2.38 vs 2.82 vs 2.89 g/dL [P = .02]; 2 weeks, 0.59 vs 0.89. vs 1.04 g/dL [P = .04]). (C-D) The adjusted mean decrement in HbA percent per day (with 95% CI) across all 3 transfusion events during the first 2 weeks after transfusion, the second 2 weeks after transfusion, and overall (change between exchanges) are shown by study arm (C) and during the first 2 weeks and second 2 weeks post-transfusion by PS-PE surface content (D). Transfusion-specific decrement per day from 2 hours to 2 weeks after transfusion is based on 28 transfusions involving 13 unique patients. Transfusion-specific decrement per day from 2 weeks to before the next transfusion included 19 total assessments from 11 unique patients. The overall change in HbA percent per day by study arm was –0.24% for those receiving ≤10-day units and –0.14% for those receiving ≥30-day units (P = .3). There were no significant differences in the change in HbA percent per day by study arm or PS-PE, but PS-PE high and ≥30-day units had greater decrements in the first 2 weeks.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodrci/1/3/10.1016_j.brci.2025.100017/2/m_brci_rci-2025-000145-gr3.jpeg?Expires=1769295929&Signature=a1Q3r~xAtup8e7dEQbRqMpBgkppkatwGKfZqfCNt24T4acp-Q6pJH12YDtRnjCS1PPD3zwiaYyvOjx9ysgAOBpUGux-ypDXs4bIuVtmJsh4hk7mC8Az8-wiWZ6hByX3Mqzhf4CvY7U4Lm-GCjX0L3ml8OuzB1HUZGd40d~TLx2Oa6x-EW58r7B-Wb4SYSyAtPPSQ~qHDa4cws3Y4aNSrYAqhFeKlSLZIBEaV2WRAx~hxdhUYMuZwTZZD60mqs8RjbZYR6koo2IEYuoxFlTenkGgvV2WatgO6c3zboc5PoiYy4ZsZ1oH0xTlpP63uRpNfJGF7ZmFT9diUJCLccZ8BbA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)