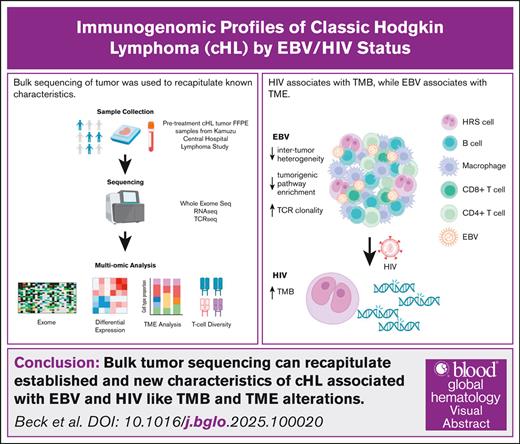
Key Points
Patient HIV status associates with tumor mutational burden, and tumor EBV status is associated with transcriptional and cell type changes.
TCR diversity primarily associates with EBV status, although HIV+ tumors trended toward further increased clonality.
Visual Abstract
Classic Hodgkin lymphoma (cHL) is a hematolymphoid neoplasm highly associated with Epstein-Barr virus (EBV), and molecular differences by tumor EBV status have been well characterized. Additionally, people with HIV (PWH) are 5 times as likely to develop cHL, and cHL in PWH is nearly exclusively EBV associated. However, there are limited published molecular characterizations of cHL arising in PWH (HIV+ cHL). This gap is particularly impactful in the Global South where cancer mortality and HIV prevalence are high. In this study, we evaluated the whole exome, transcriptome, and T-cell receptor (TCR) repertoire of an HIV-inclusive cohort of patients with cHL from Malawi. HIV+ cHL had increased tumor mutational burden compared with cHL in people without HIV (HIV– cHL). EBV positive (EBV+)/HIV– tumors exhibited a distinct transcriptional profile compared with EBV–/HIV– and EBV+/HIV+. Additionally, both EBV and HIV were associated with cellular composition changes within the tumor microenvironment, including increases in memory B cells and CD8+ T cells compared with EBV– and HIV– cHL. TCR clonality was increased in EBV+ cHL, with trend towards further increase in clonality in HIV+. Through a multiomic approach of a well-characterized, HIV-inclusive cHL cohort from one of the most resource-limited countries in the world, we were able to recapitulate known, and identify novel molecular differences by HIV and EBV status.
Introduction
Classic Hodgkin lymphoma (cHL) is a hematolymphoid neoplasm arising from dysfunctional germinal center B cells that affects >80 000 people per year worldwide.1 Although highly associated with HIV, the incidence of cHL has not decreased to the same dramatic extent as non-Hodgkin lymphomas following the introduction of antiretroviral therapy,2-4 and people with HIV (PWH) continue to have a 5- to 10-fold increased risk for developing cHL.5,6 cHL is also highly associated with Epstein-Barr virus (EBV), which is identified in ∼30% of cHL arising in immunocompetent patients, and nearly universal in PWH.7 While the global burden of cHL is expected to increase over the next decade,8 robust epidemiologic and molecular data from regions of the world with high HIV and EBV prevalence are lacking.
The tumor mass of cHL is composed primarily of an inflammatory microenvironment with scattered neoplastic Hodgkin/Reed-Sternberg (HRS) cells.9 Four histologic subtypes reflecting tumor architecture and tumor immune microenvironment (TIME) composition have been defined that differentially associate with clinical characteristics, including age, patient HIV status, and tumor EBV status.7,9,10 Additionally, changes in molecular features of cHL due to EBV have been noted, including a decrease in the number of somatic mutations within HRS cells, and increased immunosuppressive CD4+ T-cell recruitment.7 However, the characterization of HIV+ cHL has been limited to histologic characterization,11,12 despite the growing use of high-throughput sequencing in the field.13
In low- and middle-income countries (LMICs), conventional chemotherapy is available, and has been shown to be safe and effective for cHL, including in PWH.14 However, newer targeted therapies, informed by an evolving understanding of tumor-host interactions, remain largely unavailable in LMICs, where the disease burden is highest.1 Indeed, many of the genomic features and TIME characteristics of cHL with prognostic and therapeutic implications have yet to be studied and/or validated in global cohorts or in PWH.15 In addition to other factors, one reason for the lack of studies in these groups is that the advanced techniques required are typically unavailable in resource-limited settings. In preparation for future and ongoing clinical trials of immunotherapies, we aim to determine the immunogenomic profiles of cHL from an HIV-inclusive, prospectively enrolled lymphoma cohort in Malawi.
Methods
Kamuzu Central Hospital Lymphoma Study
The Kamuzu Central Hospital Lymphoma Study (ClinicalTrials.gov identifier: NCT02835911) in Lilongwe, Malawi, is a prospective observational cohort enrolling patients with newly diagnosed lymphomas since 2013.16 The Kamuzu Central Hospital Lymphoma Study was approved by the University of North Carolina Institutional Review Board and Malawi National Health Sciences Research Committee, with all patients providing written informed consent. Studies were performed in accordance with all relevant guidelines and regulations. Diagnoses were pathologically confirmed for all patients by pathologists in Malawi and hematopathologists in the United States as part of a telepathology program previously described.17,18
Ann Arbor stage was assessed by physical examination, chest X-ray, abdominal ultrasound, and bone marrow biopsy. All patients were treated with a median of 6 cycles (confidence interval [CI], 4-8) of cytotoxic combination chemotherapy, including ABVD (Adriamycin, doxorubicin, bleomycin, vinblastine, and dexamethasone)14,19; radiation and positron emission therapy were not available. PWH continued or initiated antiretroviral therapy at cHL diagnosis. Pediatric patients (aged <18 years) were followed for ≤2 years, and adult patients were followed for ≤5 years or until censored on 26 April 2024. For this study, survival data are based on the 2-year follow-up, and analyzed using survival (RRID:SCR_021137) and survminer (RRID:SCR_021094) R packages.
Pathologic diagnosis
Diagnosis was based on hematoxylin and eosin–stained formalin-fixed and paraffin-embedded (FFPE) tissue sections and immunohistochemistry (IHC) in Malawi using antibodies as previously described: CD3 (clone PS1), CD20 (clone L26), CD30 (clone 15B3), and PAX5, all from Leica Biosystems (Buffalo Grove, IL).17 Samples were shipped to the United States for genomic testing, and EBV-encoded RNA (EBER) in situ hybridization was performed on a Leica Bond platform (Leica Biosystems, Buffalo Grove, IL) according to manufacturer’s instructions. Tumor EBV status was determined by EBER expression, with >10% tumor (HRS cells) staining considered EBV positive (EBV+). However, all cases with tumor EBER expression were positive in the overwhelming majority of neoplastic cells. Histologic subtype was assigned after central review of cases in the United States by 2 hematopathologists blinded to the clinical features, as: nodular sclerosis (NS), mixed cellularity, lymphocyte rich, and lymphocyte deplete.20
IHC
IHC was conducted using antibodies: programmed death-ligand 1 (PD-L1; clone E1L3N), T-cell immunoreceptor with immunoglobulin and immunoreceptor tyrosine-based inhibitory motif domains (TIGIT; clone E5Y1W), and major histocompatibility complex class II (MHC-II; clone LGII-612.14), all from Cell Signaling Technology (Danvers, MA). For PD-L1 and MHC-II staining, samples were considered positive if proteins were expressed by neoplastic cells. TIGIT expression was scored using a published metric.21 All expression was determined by light microscopy (Olympus BX43).
Whole-exome sequencing
DNA was extracted from FFPE cHL samples (N = 23; Qiagen GeneRead DNA FFPE Kit) and whole blood pellets (QIAmp Blood Mini Kit). DNA was target-captured using the Agilent SureSelectXT All Human Exon V6, and sequenced on the NovaSeq 6000 platform. Only those that passed quality control were used in the analysis (N = 21). FASTQs were aligned by BWA-MEM (RRID:SCR_022192). BAM files were sorted and indexed using Biobambam2 (RRID:SCR_003308), and quality control by Picard (RRID:SCR_006525), MultipleMetrics (RRID:SCR_018929), and FastQC (RRID:SCR_014583). BAM realignment was done with coverageBed (RRID:SCR_006646). Variants were called by Strelka2 (RRID:SCR_005109), Cadabra (RRID:SCR_003277), and Mutect2 (RRID:SCR_000559), and merged into a single variant call format (VCF) file with vcf2maf.22 Mutations classified as silent or intronic by maftools (RRID:SCR_024519) were removed.23 Mutations were filtered using the following criteria: tumor read depth >15x, germline read depth >15x, tumor alternative allele count >5, and a variant allele frequency >1% and <10% to account for the small percentage of neoplastic cells. Median tumor depth and normal depth were 159x and 195x, respectively, after filtering.
RNA sequencing
Whole-transcriptome RNA sequencing was conducted on the same FFPE tumor samples as above. RNA was extracted (Omega Bio-Tek FFPE RNA extraction kit), cDNA libraries were prepared (KAPA HyperPrep RNA with RiboErase Kit), and strand-specific messenger RNA sequencing was performed (Illumina NovaSeq 6000 S4). Counts were generated by aligning FASTQ files with STAR (RRID:SCR_004463), and quantifying with Salmon (RRID:SCR_017036).24,25 Only samples that passed both library and sequencing quality control (Piccard 2.22.4 and FastQC 0.11.9) were included in the analysis (N = 21). Two samples were identified as outliers by quality control pipelines and principal component analysis (PCA), and were removed (N = 19). Genes with no counts across all samples were removed. Differential expression analysis and subsequent normalization was conducted using DESeq2 (RRID:SCR_015687).26 Differential pathway enrichment was conducted using the GSVA package (RRID:SCR_021058), limma (RRID:SCR_010943), and the normalized counts obtained from DESeq2 using the Hallmark Pathways data set.27-30
Cell type proportions were estimated using CIBERSORTx (RRID:SCR_016955) using the transcripts per million (TPM) counts output file from the Salmon pipeline.31 Settings were set to the default and recommended: LM22 signature matrix file, no quantile normalization, and 100 permutations.
TCR sequencing
We performed immunoSEQ Human TCRB assay according to manufacturer’s instructions (Adaptive Biotechnologies, RRID:SCR_014709) using 2 μg DNA as input wherever possible from N = 25 FFPE samples. Pooled libraries were quantified with the Collibri Library Quantification Kit (Invitrogen), and a final concentration of 1.5 pM was run on a NextSeq 500 instrument. Raw sequencing data were processed using Adaptive Biotechnologies’ pipeline. Only samples that passed Adaptive Biotechnologies’ default quality control and had >100 productive templates were included in the analysis (N = 22). Only productive templates, defined as in-frame with no stop codon, were considered. Because T-cell count impacts template count and varies widely based on FFPE tumor block, random downsampling to the lowest productive template count (n = 248) was used and averaged over n = 100 iterations.
Productive Simpson clonality was calculated as the square root of Simpson’s diversity index, where larger values represent increased clonality. Productive maximum frequency is the number of clones of the most common T-cell receptor (TCR) clone in a sample. Unique productive rearrangements is a measure of diversity, indicating the number of different TCR clones. Metrics were calculated using the amino acid sequence of the TCR using Adaptive Biotechnologies’ software. TCR overlap and epitope analysis was conducted using the immunarch package (RRID:SCR_023089).32
Results
Patient characteristics
Our cohort consisted of patients newly diagnosed with cHL from Malawi (N = 25), and included both adult (n = 18) and pediatric (n = 7) cases. The median age of the cohort was 22 years, and median progression-free survival was not reached due to censoring at 24 months (Table 1). Patients with EBV+ cHL had improved progression-free survival compared with those with EBV– cHL (hazard ratio, 0.2; P = .068). When stratified further by HIV status, there was a trend toward improved survival of patients who were EBV+/HIV– compared with patients who were EBV–/HIV– (hazard ratio, 0.3; 95% confidence index, 0.05-1.64; P = .2; supplemental Figure 1A). Most of the cohort had extensive stage (stage III-IV) disease, and limited stage (stage I-II) disease was more common in EBV+/HIV– compared with both EBV–/HIV– and EBV+/HIV+ (EBV–/HIV–: 0/9, EBV+/HIV–: 6/11, and EBV+/HIV+: 1/5). Notably, 4 out of 7 patients with limited stage disease were pediatric patients. Additionally, over half of our samples were NS subtype, including the majority of EBV+/HIV+ tumors.
Mutational profile by exome sequencing
Our initial aim was to characterize cHL genomic alterations by bulk tumor, whole-exome sequencing, recognizing that the low frequency of neoplastic cells within an abundant TIME would likely lead to the identification of sequencing artifacts. Unsurprisingly, the number of detected mutations was exceedingly large by this approach (supplemental Figure 1B), and many of the top mutated genes included frequently mutated genes (FLAGS), such as TTN and MUC16 (supplemental Figure 1D).33 We refrained from extremely strict filtering to maintain true variants with low frequency due to the nature of the tumor, and instead refined our gene targets. We first filtered for genes in the FoundationOne Heme 406 targeted gene panel,34 which is inclusive of recurrently mutated genes from a wide array of hematologic neoplasms and has been used by many prior cHL studies.35-37 With this approach, we identified more biologically relevant genes (supplemental Figure 1E), but many genes with large exonic regions dominated the landscape. Recognizing that discovery of variants by bulk sequencing would be difficult, we curated a cHL-associated gene panel based on studies using flow-sorted HRS cells, ultra-deep, and/or targeted sequencing (“curated panel,” n = 46 genes; supplemental Table 2).
Using the curated panel, samples had a median tumor mutational burden (TMB), calculated as the total number of mutations, of 2 mutations per sample. The TMB of HIV+ tumors was greater than HIV– tumors (7 vs 1.5 mutations per sample, P = .066; Figure 1A). When classified by EBV/HIV status, EBV+/HIV– tumors had the lowest TMB, in line with a previous report considering the impact of EBV on TMB (Figure 1B).7 The top 5 mutated genes were KMT2D (8/21; 38%), EP300 (6/21; 29%), CARD11 (5/21; 24%), CREBBP (5/21; 24%), and EZH2 (5/21 24%; Figure 1C). There were also no differences in TMB by subtype or age group using the curated panel (data not shown).
Mutational profile of cHL. (A) TMB of cHL-associated genes by HIV status. (B) TMB of cHL-associated genes by EBV/HIV status. TMB is calculated as the number of total mutations per sample. Horizontal lines represent group median, and P values calculated by Wilcoxon rank-sum test. (C) Oncoplot of curated panel of cHL-associated genes. Oncoplot is grouped by EBV/HIV status, and colors represent type of mutation. N/A, not available.
Mutational profile of cHL. (A) TMB of cHL-associated genes by HIV status. (B) TMB of cHL-associated genes by EBV/HIV status. TMB is calculated as the number of total mutations per sample. Horizontal lines represent group median, and P values calculated by Wilcoxon rank-sum test. (C) Oncoplot of curated panel of cHL-associated genes. Oncoplot is grouped by EBV/HIV status, and colors represent type of mutation. N/A, not available.
Assessment of TIME
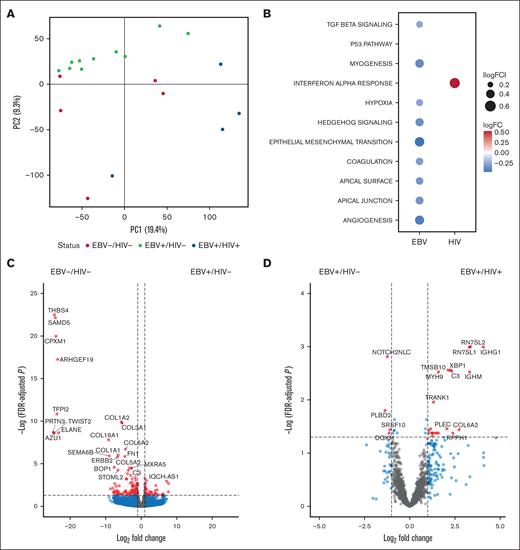
As the neoplastic HRS cells comprise a small fraction of the tumor mass, bulk RNA sequencing and differential gene expression capture the associations of EBV and/or HIV status on TIME. Of the EBV-associated cHL samples, HIV+ tumors had 26 differentially expressed genes, including XBP1 (log2FC = 2.23, Padj = .066), NCOR2 (log2FC = 1.4, adjusted P-value (Padj) = .04), and various immunoglobulin heavy chain (IGH) genes (Figure 2B; supplemental Table 4). Comparing HIV– samples, EBV+ cHL had 160 differentially expressed genes, including decreased expression of collagen and L ribosomal protein genes compared with EBV– cHL (Figure 2B; supplemental Table 5). Other genes of interest with altered expression between EBV+/HIV– and EBV–/HIV– include NCOR2 (log2FC = −1.6, Padj = .006), ARID1B (log2FC = −1.03, Padj = .006), and ZFHX3 (log2FC = −1.37, Padj = .037).
Transcriptomic profile of cHL by EBV/HIV status. (A) Principal component analysis (PCA), where each point is a sample, and color represents EBV/HIV status to highlight variation in RNA expression by EBV/HIV status. (B) Differentially enriched pathways from the Hallmark gene set database as determined by gene set variation analysis. Only significantly altered pathways (Padj < .1) are included. P values were adjusting using Benjamini-Hochberg. (C) Volcano plot of differentially expressed genes by EBV in HIV– tumors. Points indicate genes enriched in EBV–/HIV– (left) and EBV+/HIV– (right) tumors. (D) Volcano plot of differentially expressed genes by HIV in EBV+ tumors. Points indicate genes enriched in EBV+/HIV– (left) and EBV+/HIV+ (right) tumors. Cut-offs for inclusion: |Log2FC| > 1, Padj < .05. lncRNA and Y chromosome genes were excluded from volcano plots. PC1, principal component 1; PC2, principal component 2.
Transcriptomic profile of cHL by EBV/HIV status. (A) Principal component analysis (PCA), where each point is a sample, and color represents EBV/HIV status to highlight variation in RNA expression by EBV/HIV status. (B) Differentially enriched pathways from the Hallmark gene set database as determined by gene set variation analysis. Only significantly altered pathways (Padj < .1) are included. P values were adjusting using Benjamini-Hochberg. (C) Volcano plot of differentially expressed genes by EBV in HIV– tumors. Points indicate genes enriched in EBV–/HIV– (left) and EBV+/HIV– (right) tumors. (D) Volcano plot of differentially expressed genes by HIV in EBV+ tumors. Points indicate genes enriched in EBV+/HIV– (left) and EBV+/HIV+ (right) tumors. Cut-offs for inclusion: |Log2FC| > 1, Padj < .05. lncRNA and Y chromosome genes were excluded from volcano plots. PC1, principal component 1; PC2, principal component 2.
Gene set variation analysis was run to determine pathway enrichment by viral status. EBV+/HIV+ tumors had increased enrichment of the interferon-alfa response compared with EBV+/HIV– (logFC = 0.51, Padj = .01). Pathways including P53, hypoxia, and epithelial-mesenchymal transition had depleted expression in EBV+/HIV– tumors compared with EBV–/HIV– (Figure 2C).
To better observe expression of potential immune checkpoint inhibitor (ICI) targets and biomarkers within these tumors, IHC staining of PD-L1, TIGIT, and MHC-II was conducted (supplemental Table 7). PD-L1 was expressed by HRS cells in all samples. While the presence of both MHC-II and TIGIT was variable throughout the samples, expression did not associate with EBER, HIV, or other clinical demographics analyzed (supplemental Table 8).
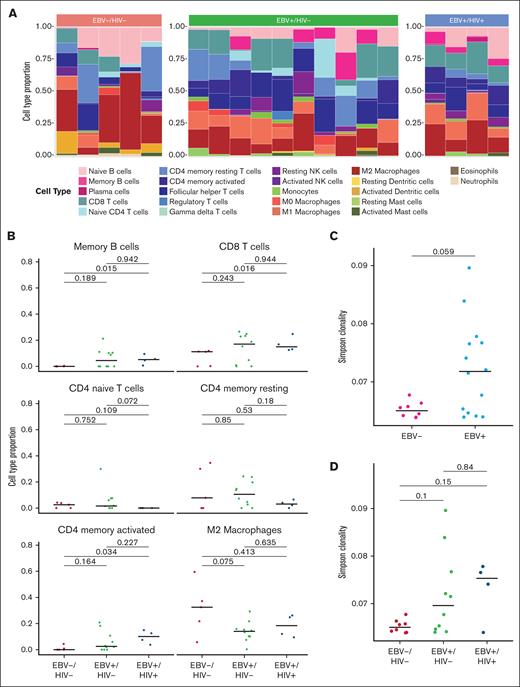
Cell type proportion analysis highlighted variation of TIME cellular composition within the 3 groups. M2 macrophage was the most represented cell type overall (Figure 3A). Additionally, EBV–/HIV– samples had a marginal increase in M2 macrophages compared with EBV+/HIV– tumors (P = .075; Figure 3B). CD8+ T cells were significantly increased in EBV+/HIV+ tumors compared with EBV–/HIV– tumors (P = .016; Figure 3B). CD4+ naive T-cell levels were higher in EBV+/HIV– than EBV+/HIV+ (P = .072; Figure 3B). While there is no apparent difference in cell type distribution by cHL subtype (data not shown), subgroup sample number limits meaningful analysis.
Immune microenvironment of cHL by EBV/HIV status. (A) Cell type proportions by EBV/HIV status estimated by CIBERSORTx. Color represents cell type. (B) Quantitative comparison of select immune cell types by EBV/HIV status. P values determined by Wilcoxon rank-sum test. (C) TCR Simpson clonality by EBV status. (D) TCR Simpson clonality by EBV/HIV status. P values determined by Wilcoxon rank-sum test. NK, natural killer.
Immune microenvironment of cHL by EBV/HIV status. (A) Cell type proportions by EBV/HIV status estimated by CIBERSORTx. Color represents cell type. (B) Quantitative comparison of select immune cell types by EBV/HIV status. P values determined by Wilcoxon rank-sum test. (C) TCR Simpson clonality by EBV status. (D) TCR Simpson clonality by EBV/HIV status. P values determined by Wilcoxon rank-sum test. NK, natural killer.
Characterization of TCR repertoire
After observing differences by bulk transcriptomics and deconvolution, and given the importance of T cells in emerging immunotherapies, we performed TCR sequencing to further elucidate these differences. TCR diversity was primarily driven by EBV status (P = .06), although HIV+ tumors also trended toward further increased clonality (Figure 3C-D). Additionally, the higher proportion of T cells observed by deconvolution did not correlate with increased diversity (supplemental Figure 2A). Clonality was not associated with clinical features, such as age (Spearman), sex (Wilcoxon rank sum), Eastern Cooperative Oncology Group (ECOG) or stage (Kruskal-Wallis). To analyze the intertumoral similarity of TCR repertoires between patients, the Morisita overlap index was calculated, and found that EBV+/HIV– repertoires were most similar to each other (supplemental Figure 2B).
Discussion
While this study corroborates known differences between EBV+ and EBV– cHL, we highlight that, even though highly associated with EBV, the molecular profile of HIV+ cHL is distinct and warrants further study. In this cohort of patients with cHL treated with ABVD, patient HIV and tumor EBV status is potentially prognostic, with EBV–/HIV– cHL associated with inferior overall survival compared with HIV+ and EBV+ tumors. Although a small cohort, this is consistent with epidemiologic studies that show in Africa, EBV+ cHL has borderline improved overall survival compared with EBV– cHL, potentially due to younger age of onset.38 TIME features were associated with tumor EBV expression, while TMB was associated with patient HIV status.
As seen in our cohort and others, EBV may act as a “first hit,” reducing the number of mutations needed to promote tumorigenesis.7 This could lead to similar tumor development and characteristics between patients, as shown with the similarity of patient tumoral TCR repertoires and transcript expression in our study. These data are also consistent with in vivo studies demonstrating that EBV effects are amplified by HIV, including increased CD8+ T-cell activation and tumorigenesis.39,40 In another study from a European cohort, the number of CD8+ T cells was the same between EBV+/HIV– and EBV+/HIV+ tumors, but displayed different expression levels of PD-1/TIGIT and CD155.41 Together with increased TMB, changes in gene expression, and increased TCR clonality within the HIV+ cHL in this cohort, our data suggest that EBV+/HIV+ cHL is distinct from EBV+/HIV– cHL, just as EBV+ cHL is distinct from EBV– cHL. Given that HIV+ cHL is nearly invariably associated with EBV, it is difficult to disentangle the effects of EBV and HIV on tumorigenesis. However, further molecular studies of cHL inclusive of PWH may shed light on relative contributions of HIV on the development of cHL.
These findings may also have future therapeutic implications, as the use of ICIs has become more common as a treatment option for relapsed/refractory cHL. Both EBV and HIV alter the expression of PD-L1 in HRS and the TIME. In our cohort, all diagnostic samples were positive for PD-L1 expression in the neoplastic cells. Additionally, retained MHC-II expression has been associated with increased response to ICIs and patient remission due to the stimulation of antitumor CD4 T cells.42 The majority of our samples have retained MHC-II expression, regardless of HIV status. A new lymphoproliferative disease treatment target, TIGIT, has also been described to be inversely correlated with PD-L1 presence in cHL, and associated with advanced disease.21 All samples in our cohort were positive for TIGIT staining; however, amount and location relative to the HRS cells varied greatly. We also did not observe a difference in gene expression between the EBV/HIV groups of these 3 molecular markers. While others have shown ICIs are safe and effective as a second-line treatment of HIV+ cHL (CATCH-IT),43 we demonstrate molecular features at initial diagnosis that others have shown predict response to therapy.44,45
Many current cHL mutational profiling and biomarker discovery studies use time- and resource-intensive sequencing methods, including targeted, ultra-deep, and/or circulating tumor DNA sequencing methods.36,37,46-48 Microdissection and flow sorting techniques provide a more detailed, in-depth view of the mutational profile of HRS cells compared with bulk sequencing, but at a much higher cost that cannot be utilized globally and/or clinically. In this study, we were able to recapitulate similar findings using less-expensive bulk sequencing. This will be particularly useful in LMICs, where the burden and mortality rates of cHL are highest.1 Additionally, all sequencing conducted here used tumor tissue, whereas new biomarker discovery methods use blood samples.44,49-51 While blood samples are noninvasive, using tissue allows for direct characterization of the tumor and TIME, and could aid in determining which treatment strategies would be most effective.
The predominance of the NS subtype in this study is an important consideration when interpreting our findings on the TIME. While NS is the most common subtype in high-income countries like the United States, HIV+ cHL is more frequently mixed cellularity.7 This distinction highlights a key difference between our study and others, which lacked representation of patients from Africa. Additionally, our cohort’s age range reflects a typical demographic seen in LMICs, providing insights more directly relevant to these regions.41-44 Many of our cases fall within the adolescent and young adult age range (15-34 years), which has unique molecular features compared with pediatric (<15 years) and older adults.52 The aim of this study was to validate immunogenomic features that are critical for improving outcomes in LMICs. As new treatment strategies and biomarkers are developed, it is essential that PWH from LMICs be included in clinical trials. This inclusive approach will ensure that current immunotherapies are evaluated in the context of the diverse immunogenomic features highlighted in this study, leading to more effective and tailored treatment strategies for all patients.
Acknowledgments
The authors sincerely thank all the patients who participated in this study and their families. The authors are grateful for the support from Kamuzu Central Hospital, the Malawi Ministry of Health, and the University of North Carolina Project-Malawi. Additional thanks to Nicholas Pankow and the Pathology Services Core at University of North Carolina (UNC), funded by National Cancer Institute (NCI) Center Core Support Grant (P30CA016086), for their technical expertise and immunohistochemistry staining.
This research was supported by the National Institutes of Health (CA016086-46S3 to UNC/Y.F., UNC-Integrated Translational Oncology Program T32-CA244125 to UNC/S.R., U54CA254564 to UNC/Y.F./S.R.) and the UNC Lineberger Comprehensive Cancer Center (Developmental Funding Award to Y.F.).
The funding agencies were not involved in the study design, data collection, analysis, interpretation, writing of the report, or the decision to submit for publication.
Authorship
Contribution: S.B., S.M.R., and Y.F. conceived and designed the analyses; S.B. analyzed and interpreted the data, with assistance from A.C., S.M.R., and A.K.V.; S.B. drafted the manuscript; S.M.R. and Y.F. revised the manuscript; M.S.P., E.K., and K.W. acquired clinical data; T.T., M.M., S.K., and Y.F. acquired pathologic data; and all authors provided edits and final approval of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Yuri Fedoriw, Department of Pathology and Laboratory Medicine, The University of North Carolina, Room 822, Brinkhous-Bullitt Building, 160 Medical Dr, Chapel Hill, NC 27514; email: yuri.fedoriw@unchealth.unc.edu.
References
Author notes
RNA raw counts and transcripts per million counts have been deposited in the Gene Expression Omnibus database (accession number GSE289903).
T-cell receptor data have been made available on ImmuneAccess (https://clients.adaptivebiotech.com/immuneaccess). Filtered exonic variants are included in this article as supplemental Data.
Code is available on request from the corresponding author, Yuri Fedoriw (yuri.fedoriw@unchealth.unc.edu).
The full-text version of this article contains a data supplement.