A few (relatively) fortunate souls with the lowest-risk disease subtypes of myelodysplastic syndromes (MDS) are likely to be alive a decade after diagnosis and are unlikely to ever progress to acute myeloid leukemia (AML), while those unlucky individuals with the highest-risk forms of disease have a life expectancy measured in months. Because the outlook for different patients with MDS varies so dramatically, prognostic tools are important to aid management decisions.
Currently, the MDS risk-stratification model most widely used in clinical practice and for clinical trial eligibility determination is the 1997 International Prognostic Scoring System (IPSS), which incorporates marrow blast proportion, marrow karyotype, and the number of peripheral blood cytopenias into a four-tier system that is predictive of survival and AML progression risk. In the last five years, investigators at MD Anderson Cancer Center developed two improved MDS risk models — a general scoring system for all MDS subtypes1 and another tool specific for IPSS lower-risk disease2 — while European investigators proposed and subsequently modified a World Health Organization (WHO) classification-based Prognostic Scoring System (WPSS).3 The MD Anderson risk models and the WPSS offer some improvements on the IPSS and complement the WHO MDS classification.
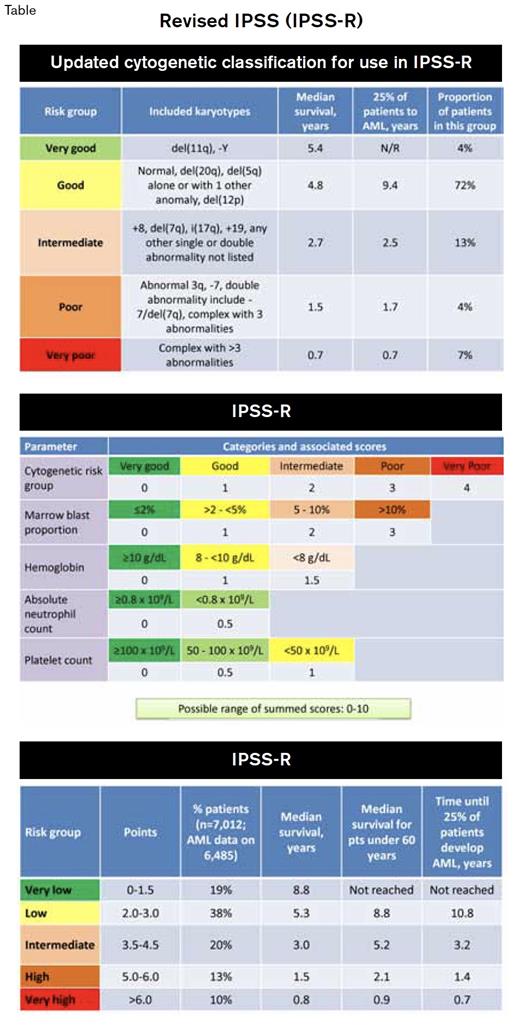
Now, 15 years after the original IPSS appeared, a large international effort coordinated by Peter Greenberg of Stanford University and sponsored by the nonprofit MDS Foundation has culminated in a Revised IPSS (IPSS-R, Tables). The IPSS-R is based on a 7,012-patient dataset, pooled from databases in 11 countries. While the IPSS-R score is generated with the same three components as the original IPSS — marrow blasts, karyotype, and cytopenias — the IPSS-R includes a broader roster of abnormal karyotypes and weighs cytogenetic results more heavily than the original IPSS did, since recent data from a large German-Austrian and MD Anderson-merged cytogenetic database indicate that the karyotype is more important than the blast proportion in determining MDS outcomes.4 Other major revisions include division of marrow blasts < 5 percent into two scoring groups (since 4% blasts are not really normal), inclusion of scores for each individual cytopenia (with minor weighing by depth of cytopenia), and expansion from four to five risk tiers. Although the IPSS-R is too complex for all but mnemonist savants to memorize, several online calculating tools and mobile apps are already available to assist hematologists who have more typical neurobiology. Check out the IPSS calculator at www.mds-foundation.org/ipss-r-calculatorwww.mds-foundation.org/ipss-r-calculator.
The IPSS-R offers several advantages that should facilitate its immediate acceptance as the heir apparent to the IPSS. Increased sensitivity to the degree of thrombocytopenia is a clear enhancement, as severe thrombocytopenia is an IPSS-independent risk validated by several independent post-IPSS analyses. Although many MDS-associated karyotypes are observed so rarely that they could not be included in the IPSS-R and remain of unclear prognostic importance, the 15 most common abnormalities are incorporated, including IPSS-opaque recurrent anomalies such as isolated del(11q) or del(12p). These changes appear to be clinically relevant: Using the IPSS-R resulted in re-categorization of 27 percent of IPSS lower-risk patients into higher-risk groups, while 18 percent of higher-risk patients were “upgraded” to a better outlook.
However, even though it represents an advance, the limitations of the IPSS-R suggest that it is likely to be a transitional tool. Like the original IPSS, the IPSS-R is only valid in adults with de novo disease at the time of diagnosis, which excludes patients with therapy-related MDS, children, and previously treated patients. The IPSS-R also does not capture the kinetics of the disease; the patient who had a normal blood count earlier in the year for an insurance examination and now has severe pancytopenia and 11 percent blasts is likely to be in a worse position than the patient with equivalent blood counts who in retrospect had eight years of mild macrocytic anemia, was never completely evaluated, and whose other counts have been slowly dwindling over the last two years.
Furthermore, neither the IPSS-R nor any of the other major risk models adequately capture the severity of patients’ comorbid conditions, which for many elderly patients are a more important determinant of life expectancy than their MDS. It is not clear whether chromosomal abnormalities detected by FISH or array-based techniques have the same implications as those included in the IPSS-R, which is based on standard G-banded karyotyping. Finally, the IPSS-R includes no molecular markers. Certain MDS-associated somatic mutations have prognostic value independent of any of the existing models,5 and understanding these is the pathway forward in MDS risk assessment. Of note, the mere presence or absence of mutations may not provide enough information; allele burden is also likely to be clinically relevant.
In Brief
Because of these limitations and the current pace of advancement in the field, it seems unlikely that it will be another 15 years before “IPSS Version 3.0” will debut. Still, the IPSS-R is a helpful advance based on a large amount of work using a database more than 8 times greater than that used for the original IPSS. This fundamental risk model will undoubtedly evolve and improve with inclusion of molecular markers and other variables. No prognostic model can provide a perfect prophecy about the appointed time of a patient’s death — nor, given human nature with its abhorrence of determinism and individual psychologic need for exceptionalism, would we want it to. Prognosis also is intimately dependent on available therapies, and new drugs that can change the natural history of the disease may invalidate prognostic scoring systems: Remember that once acute promyelocytic leukemia was the deadliest form of AML, and then along came all-trans-retinoic acid and arsenic trioxide, which changed everything.
References
Competing Interests
Dr. Steensma indicated no relevant conflicts of interest.