The Question
What is the optimal, upfront management of mantle cell lymphoma (MCL)?
Our Response
MCL is a rare type of lymphoma, representing about 6 percent of all cases of non-Hodgkin lymphoma.1 Median age at diagnosis is 68 years, and the vast majority of patients are diagnosed with advanced-stage disease. Patients with MCL typically present with generalized lymphadenopathy and extranodal involvement, particularly in the bone marrow, spleen, and gastrointestinal tract. Cyclin D1 expression as a result of t(11;14)(q13;q32) translocation is present in more than 95 percent of cases. Notably, MCL has a poor prognosis, though the median overall survival (OS) has improved over time and is now approximately seven to eight years.
Given the small number of randomized clinical trials in previously untreated patients with MCL, none of which have demonstrated an improvement in OS, the optimal initial approach to MCL has not been clearly established. Subsequently, patient participation in well-designed clinical trials is highly recommended. This article will focus on treatment strategies that have been shown to offer the best chance of prolonged remission.
A Watch-and-Wait Strategy Is Appropriate for a Subset of Patients
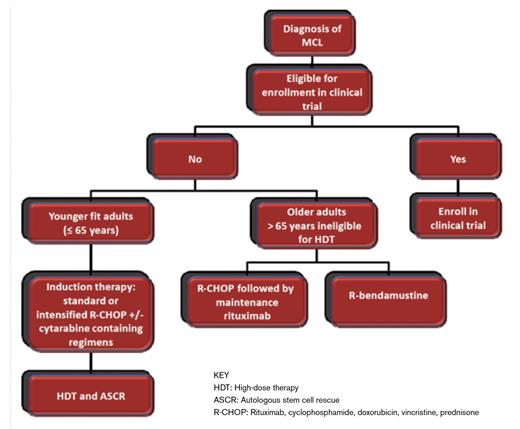
Given the burden of disease, the majority of patients with MCL require therapy at diagnosis. However, a subset of patients with splenomegaly and circulating lymphoma cells with a low ki-67, typically without significant lymphadenopathy, may have an indolent course. Data from Weill Cornell Medical Center also suggest that, for selected patients with asymptomatic disease, there is no survival disadvantage to expectant management.2 For those requiring therapy, patients may be divided into categories of young and fit (≤ 65 years without significant comorbidity) or older (> 65 years with inability to tolerate high-dose therapy).
Young and Fit Adults with MCL
Although the addition of rituximab to conventional chemotherapy regimens has significantly improved prognosis in nearly all subtypes of B-cell non-Hodgkin lymphoma, its addition to cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) in MCL has not translated into improved outcomes. The German Low Grade Lymphoma Study Group found that, due to the short durability of the response, rituximab with CHOP (R-CHOP) compared with CHOP did not improve progression-free survival (PFS) despite an improvement in overall response rate (94% vs. 75%).3 Given the disappointing outcome with R-CHOP therapy alone in younger patients, emphasis has been placed on intensification of induction therapies and consolidation strategies with high-dose therapies followed by autologous stem cell rescue (ASCR).
A randomized trial comparing myeloablative radiochemotherapy and ASCR versus maintenance interferon-α in patients ≤ 65 years achieving a partial or complete response with CHOP-like induction, with or without rituximab, demonstrated an improved PFS in the radiochemotherapy arm (median PFS, 39 months vs. 17 months) though there was no OS difference.4 This study is the basis for adoption of high-dose therapies and ASCR as consolidative strategies in younger patients. The Nordic Lymphoma Group in the MCL2 study adopted a cytarabinecontaining induction regimen consisting of six cycles of dose-intensified CHOP alternating with high-dose cytarabine with rituximab in cycles two through six, followed by highdose therapy (HDT) and ASCR. The four- and 10-year PFS were highly encouraging at 73 percent and 55 percent, respectively.5 A median event-free survival of 84 months was reported by the French group in a phase II trial using an induction regimen consisting of three cycles of CHOP and three cycles of rituximab with DHAP (dexamethasone, highdose cytarabine, and cisplatin) followed by HDT and ASCR.6 Preliminary results of a randomized phase III trial comparingsix cycles of CHOP to R-CHOP/rituximab with DHAP, suggest both a PFS and OS advantage for patients receiving the intensified regimen.7 In terms of post-transplantation strategies, preliminary results from a randomized study from Europe demonstrated an improved three-year event-free survival of 88 percent versus 73 percent in patients receiving maintenance rituximab versus observation.8
Another approach to increase the durability of remission in MCL patients uses intensified doses of chemotherapy including cytarabine without stem cell transplantation. A phase II trial of R-HyperCVAD (rituximab, fractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone alternating with high-dose methotrexate and cytarabine) by the MD Anderson Cancer Center group showed a high complete remission rate of 87 percent and three-year failure-free survival of 64 percent. However, this therapy has significant toxicity, with 8 percent treatment-related mortality.9 The regimen has been tested in the cooperative group setting by the Southwest Oncology Group, with demonstrated three-year PFS and OS of 66 percent and 81 percent respectively.10 Of note, 39 percent of patients were unable to complete all therapy due to significant myelosuppression and infection. Therefore, this therapy should be reserved for young and fit patients with close monitoring for toxicity. Finally, a comparative effectiveness study from the National Comprehensive Cancer Network NHL database comparing initial MCL therapy in younger patients demonstrated that R-CHOP alone was inferior to aggressive therapies such as R-HyperCVAD or R-CHOP followed by HDT and ASCR.11
In general, we favor an intensive treatment approach consisting of an induction regimen followed by HDT and ASCR for patients who are 65 years or younger without significant comorbidity. The optimal induction chemotherapy has not been established, and options include standard or intensified R-CHOP with or without the addition of cytarabinecontaining regimens. Post-transplant maintenance rituximab may be considered, but it is associated with neutropenia and risk of sinopulmonary infections.
Older Adults with MCL
The use of dose-intensified, cytarabine-containing induction regimens followed by HDT and ASCR is not feasible for most elderly patients, due to excessive toxicity. Therefore, treatment strategies focus on regimens that improve PFS while minimizing toxicity. Results of a double randomized trial comparing R-CHOP versus R-FC (rituximab, fludarabine, and cyclophosphamide) in patients older than 60 years who were ineligible for HDT, with second randomization of responders (complete or partial response) to either maintenance rituximab or maintenance interferon-α showed a clear OS benefit as well as less toxicity for R-CHOP compared with R-FC. The remission duration was also doubled in the maintenance rituximab versus the interferon-α arm. Moreover, among patients who had a response to R-CHOP induction, maintenance rituximab significantly improved OS when compared with maintenance interferon-α (4-year OS, 87% vs. 63%), while it had no influence in the group that received R-FC induction.12
Bendamustine is also a highly active drug in MCL. When compared to R-CHOP, bendamustine plus rituximab (BR) resulted in an improved PFS of 35 months compared to 22 months in a randomized study from Europe.13 In early results from a small study in the upfront setting, the combination of lenalidomide plus rituximab yielded overall and complete response rates of 92 and 64 percent respectively with a two-year PFS of 85 percent.14 In a randomized study comparing bortezomib, rituximab, cyclophosphamide, doxorubicin, and prednisone (VR-CAP) to R-CHOP in transplant-ineligible patients, VR-CAP resulted in a superior median PFS of 24.7 months compared with 14.4 months with R-CHOP.15 However, there were significantly increased rates of toxicity with VR-CAP, and it is not clear whether its superior PFS will be sustained in the setting of rituximab maintenance after R-CHOP. An ongoing intergroup study in the United States is currently comparing BR with or without bortezomib followed by random assignment to maintenance rituximab with or without lenalidomide. Until these data are mature, we favor R-CHOP or BR therapy followed by maintenance rituximab as upfront therapy in older patients with MCL.
References
Author notes
This article was rewritten in 2016 when this article was included in the Ask the Hematologist Compendium 2010-2015Ask the Hematologist Compendium.
Competing Interests
Dr. Odejide and Dr. LaCasce indicated no relevant conflicts of interest.