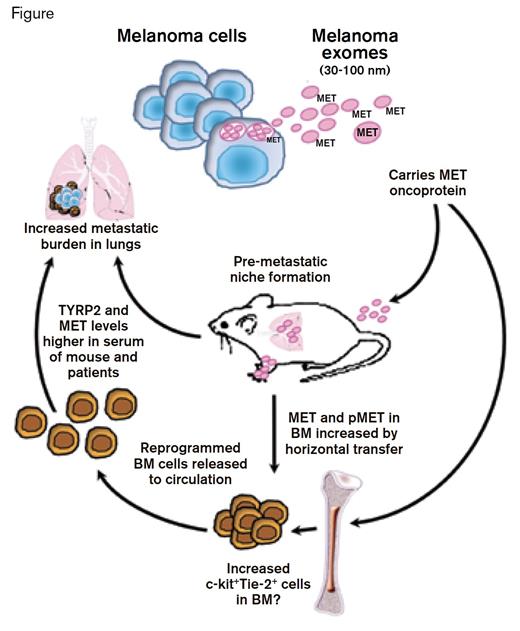
Tumor metastasis is a hallmark of aggressive disease and is usually associated with an unfavorable prognosis. The mechanism by which a tumor develops metastatic potential is thought to result primarily from acquisition of cell-autonomous (intrinsic) properties that mediate the selection and outgrowth of a clone capable of vascular dissemination. According to this hypothesis, accumulation of somatic mutations that affect the genetic and epigenetic properties of the cell underlies clonal evolution. However, metastatic properties could be acquired through other mechanisms. For example, cell-cell fusion between tumor cells and bone marrow macrophages has been proposed as an extrinsic mechanism for acquiring the pathologic properties necessary to induce metastasis,1 and now, a study from the laboratory of David Lyden of Weill Cornell Medical College in New York suggests another mechanism by which an extrinsic process may contribute to the development of metastatic disease. Tumor cells are known to release membrane-bound nano-sized vesicles (30-100 nm) called exosomes that traffic protein and RNA between cells (Figure). Tumor exosomes are readily detected in body fluids including serum, saliva, urine, and breast milk. The current study presents evidence of cellular reprogramming of bone marrow cells by tumor-derived exosomes.
Peinado et al. demonstrate that exosomal transfer of the oncoprotein MET from melanoma cells to bone marrow progenitor cells induces release of bone-marrow-derived progenitor cells. This process contributes to the switch from localized disease to disseminated disease because the pro-angiogenic and pro-vasculogenic properties of the released bone marrow cells provide a niche for expansion of disseminated melanoma cells (Figure). To decipher the molecular and cellular events involved in this process, the authors used a murine model. Cell-free exosomes were derived from the highly metastatic mouse melanoma cell line, B16-F10. Exosome infusion followed by injection of melanoma cells resulted in an increase in the burden of pulmonary metastases. Further experiments showed that intravenous injection of exosomes increased the proportion of c-kit+/Tie-2+ progenitors in the bone marrow and induced their release into the circulation. Based on increased MET protein and MET phospho-protein expression in the bone marrow cells, these events appeared to be mediated by direct transfer of MET (a receptor tyrosine kinase) from the tumor exosomes to bone marrow cells. Intriguingly, these alterations in MET expression were durable as the bone marrow cells retained their “educated” metastatic phenotype after adoptive transfer to a naive host. Finally, the authors showed that knockdown of Rab27a, a protein important for exosome biogenesis, decreased exosome secretion by mouse and human cell lines and consequently blunted the metastatic potential of the malignant cells.
The murine studies were complemented by a correlative analysis of patient specimens that showed the presence of characteristic melanoma markers, TYRP2 and MET, in serum-derived exosomes from patients with regional and distant metastatic disease. Analogous to the murine studies, patients with stage 3 and 4 melanomas also demonstrated higher levels of activated phospho-MET in tumor exosomes, and circulating bone marrow progenitor cells from patients had higher expression of phospho-MET when compared with cells from healthy volunteers. A direct correlation was observed between MET expression in tumor exosomes and subsequent development of metastasis in patients, suggesting that quantitation of MET expression in serum exosomes might serve as a predictive marker of metastatic disease.
In Brief
While the specifics are intriguing, more broadly, the study hints at the complexity of the cargo carried by tumor exosomes, suggesting an enormous potential to directly influence the biology of the tumor microenvornment. The experiments also identify another part of the multi-compartmental physiology of metastatic cancer by demonstrating that exosome trafficking alters the malignant phenotype and transcends conventional paracrine signaling by direct cytoplasmic transfer of a receptor tyrosine kinase. Even though experimentally feasible, global interference with exosome release as a constitutive cellular function is too broad a therapeutic target. Going forward, we can anticipate that discovery-driven proteomic or transcriptomic analyses will dissect the molecular events that result from vesicle trafficking in the tumor and bone marrow microenvironment and lead to the identification of more suitable therapeutic targets.
Peinado and colleagues provide persuasive evidence that tumor-derived exosomes promote melanoma metastasis by activating cells in the bone marrow and prompting the formation of a “pre-metastatic” niche. Questions remain unanswered about the mechanism by which c-kit/Tie-2–expressing cells shape the microenvironment in the metastatic target tissue and about other cellular processes that are affected by the exosome transfer process. Nevertheless, this imaginative study has both diagnostic and therapeutic implications and should stimulate further research into the role of exosomes and their cellular targets in the bone marrow during the evolution and progression of cancer.
References
Competing Interests
Drs. Sahoo and Kurre indicated no relevant conflicts of interest.