Acute myeloid leukemia (AML) is the archetypal neoplasm for the characterization of cancer genomes. Dozens of recurrent, AML-associated chromosomal rearrangements and somatic point mutations are now recognized and these influence clinical risk stratification. However, such genetic information does not currently affect treatment decisions beyond identification of those patients with AML who have a reasonable chance of cure with high-dose chemotherapy alone versus those whose odds are poor enough to justify the risk of an allogeneic stem cell transplant.1 Most patients diagnosed with AML in 2012 will be treated with the same anthracycline-cytarabine combination regimens that were developed in the 1970s, or else (if the patient is especially old or infirm) with lower-intensity palliative strategies that improve median survival by only a couple of months.
In order to better understand how the full complement of AML-associated point mutations might influence outcomes in a uniformly treated cohort of patients, Ross Levine’s laboratory at Memorial Sloan-Kettering Cancer Center assessed the mutational status of 18 genes in samples from 502 patients between ages 18 and 60 (398 patients in the test cohort, 104 in the validation cohort) from among the 657 participants enrolled in the Eastern Cooperative Oncology Group (ECOG) E1900 randomized clinical trial.2 The ECOG E1900 study demonstrated superiority of daunorubicin 90 mg/m2 /day compared with 45 mg/m2 /day as part of the 3 and 7 induction regimen, at least for patients with favorable or intermediate-risk cytogenetics. According to the E1900 trial results, which were published in 2009, the higher dose of daunorubicin was associated with a superior rate of complete remission (71% vs. 57%) and improved overall survival (median, 23.7 vs. 15.7 months) compared with the lower dose, without increasing the rate of serious adverse events.2
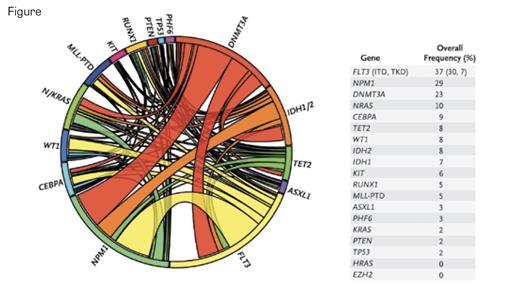
This Circos diagram depicts the relative frequency and pairwise co-occurrence of mutations in patients with newly diagnosed AML who were enrolled in the Eastern Cooperative Oncology Group E1900 clinical trial. The length of the arc corresponds to the frequency of mutations in the first gene, and the width of the ribbon corresponds to the percentage of patients who also had a mutation in the second gene. Pairwise co-occurrence of mutations is denoted only once, beginning with the first gene in the clockwise direction. The diagram also shows the frequency of mutations in the test cohort. N Engl J Med. 2012;366:1082.
This Circos diagram depicts the relative frequency and pairwise co-occurrence of mutations in patients with newly diagnosed AML who were enrolled in the Eastern Cooperative Oncology Group E1900 clinical trial. The length of the arc corresponds to the frequency of mutations in the first gene, and the width of the ribbon corresponds to the percentage of patients who also had a mutation in the second gene. Pairwise co-occurrence of mutations is denoted only once, beginning with the first gene in the clockwise direction. The diagram also shows the frequency of mutations in the test cohort. N Engl J Med. 2012;366:1082.
Levine and his colleagues found that 97 percent of the samples from E1900 patients had at least one identifiable somatic mutation, the most common of which were FLT3 (37%), NPM1 (29%), DNMT3A (23%), and NRAS (10%). The major findings of the new analysis are that FLT3 internal tandem duplication (ITD), partial tandem duplication in MLL, and mutations in ASXL1 and PHF6 were independently associated with reduced overall survival in the E1900 cohort, while CEBPA and IDH2 predicted better outcomes. NPM1 mutations were only favorable if present in conjunction with an IDH1/2 mutation. Of more immediate clinical relevance, higher-dose daunorubicin improved survival among patients with DNMT3A or NPM1 mutations or MLL translocations but did not influence survival among patients without such mutations. Complex interactions between the various mutations appear important but require further study.
In Brief
Currently, the National Comprehensive Cancer Network (www.nccn.org) AML Clinical Practice Guideline recommends evaluation of the mutation status of only four genes at the time of diagnosis: NPM1, FLT3,CEBPA, and KIT. Few clinical pathology laboratories offer additional mutation testing beyond this short list, even for the subset of genes that are relatively commonly mutated in AML (e.g., DNMT3A andIDH1/2) and that were known to influence outcomes well before the new E1900 molecular profiling study. Additional mutational profiling seems likely to become more widely available in the near future, especially if other clinical groups are able to confirm that molecular analysis in AML can influence more than the single binary treatment decision about whether to proceed with transplantation. Next-generation sequencing approaches have dramatically decreased the cost of sequencing, enabling the profiling of a larger panel of mutations at a low cost. Nevertheless, obstacles remain, such as intellectual property claims for mutation testing of FLT3 and other genes and the logistics of returning such information in real-time to clinicians. Still, deep genetic characterization of patients enrolled in the numerous ongoing treatment trials of novel agents by the cooperative groups and other institutional networks promises to identify molecular subgroups with particular therapeutic sensitivities, hopefully making therapeutic calculations in AML more complex than the current basic math of adding 3 to 7.
References
Competing Interests
Drs. Steensma and Ebert indicated no relevant conflicts of interest.