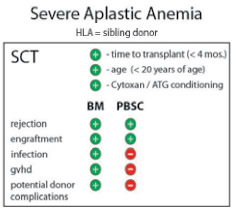
Severe aplastic anemia (SAA) is a potentially fatal, acquired disease characterized by pancytopenia and a hypoplastic bone marrow. In most cases, the disease is idiopathic, although compelling indirect evidence suggests that the attack on bone marrow cells is immune-mediated. Durable remission can be achieved using immunosuppressive therapy, but allotransplantation is the only truly curative treatment. Optimal outcome depends upon the age of the patient and the availability of an HLA-matched sibling donor, meaning that the minority of patients with SAA undergo allogeneic stem cell transplantation. Still, with more than three decades of aggregate experience and thousands of patients treated, long-term outcome for this group is the envy of the transplant field. Taking advantage of successive improvements in HLA matching, transfusion practices, and infection prophylaxis, survival rates after matched sibling transplantation approach 90 percent and are higher yet in very young patients. Without the need to eradicate an underlying malignancy, the conditioning strategies are sub-myeloablative and avoid the toxicity associated with high-dose, total-body irradiation. Treatment is carefully tailored to minimize rejection, resulting in low rates of graft-versus-host disease (GVHD) when bone marrow is used as the stem cell source.1 Prior studies suggested an improved outcome for very young SAA patients (those <20 years old) when bone marrow (as opposed to peripheral blood) was used as the stem cell source. Now, a large retrospective, multinational study demonstrates, with a wide margin of statistical significance, that such findings hold true across all age groups.
By way of background, the Center for International Blood and Marrow Transplant Research (CIBMTR) has reported that among patients 20 or older undergoing allogeneic transplantation between 2005 and 2009, 80 percent received peripheral blood stem cell (PBSC) grafts. Of course, the enthusiastic and rapid embrace of PBSC grafting during the preceding decade largely reflects allogeneic transplants undertaken to treat hematologic malignancies. The purported advantages of using PBSCs (e.g., rapid engraftment, reduced incidence of rejection), however, appear to have been extrapolated to SAA as unpublished data from both CIBMTR and the European Group for Blood and Marrow Transplantation (EBMT) registries2 indicate that up to 60 percent of patients transplanted for SAA in 2009 received PBSCs.
Predictors of Outcome After Matched Sibling Transplant for Patients With Severe Aplastic Anemia.
Predictors of Outcome After Matched Sibling Transplant for Patients With Severe Aplastic Anemia.
On behalf of the Aplastic Anemia Working Party of the European Group for Blood and Marrow Transplantation (WPSAA-EBMT), Bacigalupo and colleagues recently summarized outcomes in more than 1,800 patients across 305 centers who underwent matched sibling transplantation for SAA between 1999 and 2009. The analysis revealed highly significant negative predictors of outcome, including patient age >20 years, prolonged interval between diagnosis and transplantation, and conditioning strategies not including cyclophosphamide or anti-thymocyte globulin. But most striking was the negative impact observed when mobilized peripheral blood was used as the stem cell source (Figure). The authors were careful to investigate potential bias attributable to center effects, demographics, and conditioning treatment. To exclude confounders, they assigned factor scores and developed cohorts with substantial survival differences. Remarkably, across three cohorts and in multivariate analysis, PBSC as the stem cell source resulted in significantly inferior survival with a concurrent two-fold increase in GVHD. These results follow up on an earlier study of more than 600 patients that showed the increased risk of GVHD for younger recipients of PBSC grafts2 and comes on the heels of another study that showed a survival advantage for BM stem cell recipients after matched unrelated transplantation.3
In Brief
What then are the lingering reasons for advocating the use of peripheral blood stem cell grafts in SAA? Some are intuitive, but unproven. While the abundance of progenitors in mobilized PBSC collections can hasten hematologic recovery, early engraftment did not translate into appreciable differences in transplant-associated mortality (Figure). Rejection was not different between the two stem cell sources, and the use of stem-cell-mobilized peripheral blood grafts did not decrease donor morbidity, a widely held perception (Figure). Infection rates actually increased after PBSC transplantation (Figure), leaving, broadly speaking, logistical preference and procedural ease as deciding factors. What clinical indications are left for using mobilized peripheral blood grafts in SAA? One example may be transplantation of patients following rejection of their first graft. Another reason could be specific donor health risks that preclude the use of general anesthesia that is required for a bone marrow harvest procedure. As always, clinical equipoise is key. However, evidence from multiple sources and across several large studies now uniformly favors the use of bone marrow as the stem cell source for patients with SAA undergoing allogeneic bone marrow transplantation.
References
Competing Interests
Dr. Kurre indicated no relevant conflicts of interest.