The protean clinical manifestations of iron overload observed in patients with either primary or secondary hemochromatosis reflect the toxic effects of excess-free iron that cause end-organ damage through oxidative injury to lipids, proteins, and DNA. Phlebotomy and iron chelators are effective in preventing and, in some cases, reversing end-organ damage caused by iron overload. However, not all patients are good candidates either for phlebotomy because of anemia or for iron chelators because of adverse effects or expense. Therefore, new approaches to preventing and treating iron overload are needed, and understanding the fundamentals of iron metabolism and the pathophysiology of iron overload is of paramount importance if novel therapies are to be developed successfully.
Recent studies from a number of investigators (many of whom are ASH members) have delineated how nature handles iron. The story of hepcidin, a peptide hormone that mediates iron homeostasis, is among the most fascinating investigative triumphs in hematology. By inducing endocytosis and degradaton of its receptor, ferroportin, the “door” through which cells export iron, hepcidin controls plasma-iron concentration by inhibiting both dietary iron absorption and release of recycled iron from macrophages. Hepcidin deficiency underlies or contributes to iron overload in hereditary hemochromatosis, thalassemia, and a number of other disease states. Several investigations have suggested that replacement of hepcidin would be a rational approach to the prevention and treatment of iron overload in these disorders.1-3 However, the use of recombinant hepcidin as a replacement therapy in hepcidin-deficient conditions has major limitations. Generation of a correctly folded, full-length hepcidin protein is challenging and expensive as the 25 amino acids molecule has four disulfide bonds that are required for maintenance of its tertiary structure. Further the protein’s molecular mass of 2.7 kDa limits GI absorption, and in vivo, the half-life of the renally excreted protein is short. Based on structure/function studies of hepcidin/ferroportin, Preza et al. in the laboratories of Elizabeth Nemeth and Tomas Ganz at UCLA developed a series of seven- to nine-amino-acid peptides, “minihepcidins,” that mimic the activity of hepcidin in cell-based bioassays and in mice. Their findings establish the feasibility of small, drug-like hepcidin mimetics for the treatment of iron overload disorders due to hepcidin deficiency.
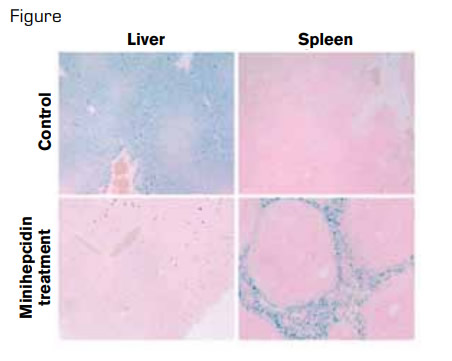
Minihepcidin Treatment Prevents Hemochromatosis Phenotype in Hepcidin Knockout Mice. In hereditary hemochromatosis, because of hepcidin deficiency, iron is excessively exported into plasma, both from enterocytes, which absorb dietary iron and from macrophages, which recycle iron from old red blood cells. This process results in iron overload of hepatocytes in the liver and iron-deficient spleen macrophages, as shown in the top row of the figure for knockout mice treated with solvent only. In the bottom row, two weeks of daily minihepcidin injections prevented liver iron accumulation and caused normalization of iron stores in spleen macrophages.
Minihepcidin Treatment Prevents Hemochromatosis Phenotype in Hepcidin Knockout Mice. In hereditary hemochromatosis, because of hepcidin deficiency, iron is excessively exported into plasma, both from enterocytes, which absorb dietary iron and from macrophages, which recycle iron from old red blood cells. This process results in iron overload of hepatocytes in the liver and iron-deficient spleen macrophages, as shown in the top row of the figure for knockout mice treated with solvent only. In the bottom row, two weeks of daily minihepcidin injections prevented liver iron accumulation and caused normalization of iron stores in spleen macrophages.
The investigators targeted the extracellular loop of ferroportin that is involved in hepcidin binding. By identifying hepcidin residues critical for binding to ferroportin, computer modeling allowed the design of minihepcidins, seven to nine amino acids long. The capacity to induce ferroportin degradation was used to screen the synthesized peptides for functional activity. Aromatic and hydrophobic side chain interactions and cysteine thiols necessary for disulfide exchange with ferroportin were necessary for maximal activity. Pegylation increased solubility and use of unnatural D-amino acids enhanced resistance to proteolysis. These minihepcidins given intraperitoneally or orally by gavage after conjugation to bile acids or palmitate caused hypoferremia in mice, and they prevented hepatic iron overload in hepcidin-1 knockout mice (Figure).
In Brief
These careful, rigorous studies support the therapeutic utility of minihepcidins to block hyperabsorption of dietary iron in patients with hemochromatasis. It is not clear whether minihepcidins would “unload” iron from affected organs in patients with hemachromatosis, but conceivably, they could redistribute iron from parenchymal cells to macrophages. Such redistribution is potentially therapeutic as macrophages are more resistant to the toxic effects of iron than organ tissues such as hepatocytes and cardiac myocytes that are commonly affected in patients with hemochromatosis.4 These promising results, based on understanding nature’s way of handling iron, may bring to the clinic new agents for treating iron overload by locking and degrading the “iron door.”
References
Competing Interests
Dr. Vercellotti indicated no relevant conflicts of interest.