Kamykowski J, Carlton P, Sehgal S, et al. Quantitative immunofluorescence mapping reveals little functional coclustering of proteins within platelet α-granulesQuantitative immunofluorescence mapping reveals little functional coclustering of proteins within platelet α-granules. Blood. 2011;118:1370-1373.
Battinelli EM, Markens BA, Italiano JE Jr. Release of angiogenesis regulatory proteins from platelet alpha granules: modulation of physiologic and pathologic angiogenesisRelease of angiogenesis regulatory proteins from platelet alpha granules: modulation of physiologic and pathologic angiogenesis. Blood. 2011;118:1359-1369.
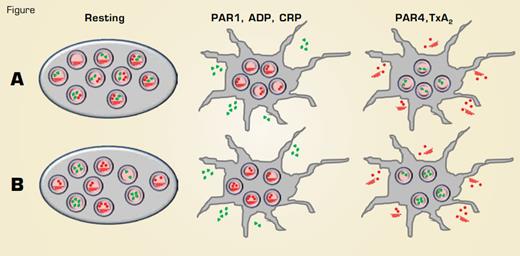
Agonist-Selective Release of Contents From α-Granules. Model A proposes that granule cargos are distributed in a random manner among α-granules, not in specific subpopulations. Cargo may be clustered with α-granules, given the appearance of segregation and enabling release of specific contents in response to different agonists. Model B postulates that different granule contents are segregated into discrete subpopulations of α-granules that are released in response to different agonists. Angiogenic factors are depicted in green and anti-angiogenic factors depicted in red.
Agonist-Selective Release of Contents From α-Granules. Model A proposes that granule cargos are distributed in a random manner among α-granules, not in specific subpopulations. Cargo may be clustered with α-granules, given the appearance of segregation and enabling release of specific contents in response to different agonists. Model B postulates that different granule contents are segregated into discrete subpopulations of α-granules that are released in response to different agonists. Angiogenic factors are depicted in green and anti-angiogenic factors depicted in red.
For the past 50 years, platelet biologists have looked at platelets under the electron microscope and have observed three types of granules: dense granules, lysosomes, and α-granules. The α-granules outnumber the other two granule types by an order of magnitude and contain hundreds of different protein cargos. Some of these cargos have opposing activities, such as prothrombotic and anti-thrombotic or angiogenic and anti-angiogenic. How can cargos with opposing activities efficiently elicit physiological responses? One possibility is that α-granule contents are released indiscriminately and the physiological activity of platelet releasate is dictated solely by stoichiometry and kinetics of their activities on target receptors. However, recent evidence suggests that the release of specific contents from platelets is controlled at the level of the α-granule exocytosis. These studies indicate that specific platelet cargos are stored in different subsets of α-granules that are released only in response to particular agonists. Alternatively, cargos may be randomly distributed among granules but segregated within the granule and released preferentially in response to different agonists (Figure).
A recent series of articles published in Blood address this controversy. Kamykowski et al. from Little Rock, AR, used confocal microscopy with three-dimensional reconstruction and quantitative analysis to assess the co-distribution of 15 different α-granule cargos. The frequency of co-distribution of the different granule pairs followed a Guassian distribution, consistent with the interpretation that cargos are randomly distributed among α-granules. The authors attributed segregation of α-granule contents previously observed by investigators to compartmentalization of cargo within single α-granules (Figure A, Resting). Two other reports, however, demonstrated that angiogenic factors localize to different α-granules and are released upon stimulation by different agonists (Figure B). Battinelli et al. from Joseph Italiano’s lab in Boston demonstrated that the angiogenic factor vascular endothelial growth factor (VEGF), but not the anti-angiogenic factor endostatin, is released from granules following exposure of platelets to ADP. In response to thromboxane A2, endostatin is released, but VEGF is not. Chatterjee et al. from Stockholm also showed that ADP is a potent secretagogue for VEGF and other angiogenic factors but not for antiangiogenic factors such as endostatin. Further studies by this group were consistent with previous results demonstrating that PAR1 agonists release angiogenic factors, while PAR4 releases anti-angiogenic factors.1,2 Differential secretion of granule contents in response to different agonists is difficult to reconcile with the hypothesis that cargo proteins are randomly distributed among α-granules. A model including partial selective release of specific granule cargos from individual platelets has been proposed (Figure A).
In Brief
Over the past decade, we have come to expect a lot more of platelets. In addition to their established role in hemostasis and thrombosis, they have been credited with roles in inflammation, atherosclerosis, angiogenesis, wound healing, antimicrobial host defense, and malignancy. Central to many of these activities is the function of α-granules. The mechanisms that enable platelets to control the release of α-granule contents that, in some cases, have opposing activities are unknown. In many nucleated cells, different subtypes of granules contain different granule cargos that are released in response to different agonists. The studies by Battinelli et al. and Chatterjee et al. indicate that the same is true in platelets. However, the study by Kamykowski et al. suggests a random distribution of cargo among platelet granules. How will this controversy be resolved? Future studies of α-granule formation may demonstrate that the delivery of cargos into nascent α-granules at the trans Golgi network occurs in a random fashion, or instead that this delivery is patterned to provide for the formation of α-granule subtypes containing different cargos. A better understanding of the distribution of the machinery that controls secretion, such as SNAREs and Rab proteins, could reveal the mechanism of differential α-granule release. Alternatively, understanding the dynamics of the fusion pore may reveal how platelets manage the release of cargos with opposing functions.
References
Competing Interests
Dr. Flaumenhaft indicated no relevant conflicts of interest.