Streptococcus pyogenes infection is associated with disease ranging from mild pharyngitis to severe, potentially fatal events, including streptococcal toxicshock syndrome. Additionally, rheumatic fever and post-streptococcal acute glomerulonephritis continue to be common in developing nations. Streptococcal M protein, which was discovered by Rebecca Lancefield in 1928,1 is a 48-kDa fibrillar, surface-associated major virulence factor in streptococcal infections. Soluble M protein is produced by proteolytic cleavage at the cell surface by neutrophil proteases. There are more than 100 types of M proteins, of which the M1 type is the most common. M1 protein binds fibrinogen, which results in vascular leakage and tissue injury, which are hallmarks of toxic-shock syndrome. The M1-fibrinogen complex binds β2 integrins on neutrophils, resulting in the secretion of heparin-binding protein (also known as azurocidin), which is an important mediator of the vascular pathology associated with streptococcal infection.2 The M1-fibrinogen interaction also results in integrin-dependent platelet activation, leading to additional pro-inflammatory events.
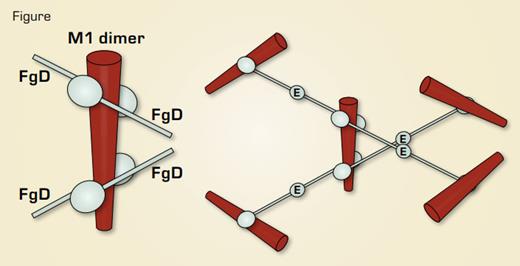
Streptococcal M1 Protein – Fibrinogen Complex. Left, schematic of the x-ray structure of a 17-kDa M1 fragment complexed to fibrinogen fragment D. Right, model of the M1-fibrinogen complex based on the D-E-D fibrinogen symmetry.
Streptococcal M1 Protein – Fibrinogen Complex. Left, schematic of the x-ray structure of a 17-kDa M1 fragment complexed to fibrinogen fragment D. Right, model of the M1-fibrinogen complex based on the D-E-D fibrinogen symmetry.
To address the question of how the M1-fibrinogen complex produces neutrophil activation, Macheboeuf et al. determined a 3.3 Å crystal structure of a 17-kDa fragment of M1 in complex with the 86-kDa fibrinogen fragment D. In the structure, M1 forms a dimer to which four fragment D molecules are bound (as shown schematically in the left of the Figure). Fibrinogen has two D domains and a central E domain, producing a symmetrical D-E-D molecule. After proteolytic cleavage of fibrinogen by thrombin and intermolecular association of the D and E domains, the D-E-D symmetry is the basis for the polymerization of fibrin to form the three-dimensional clot network. Macheboeuf et al. propose that streptococcus takes advantage of the symmetry of fibrinogen by using M1 protein to form its own Tinkertoy-like version of a three-dimensional fibrinogen network (as shown in the right of the Figure). Transmission electron microscopy of M1-fibrinogen complexes was consistent with this network structure. In their model, binding and clustering of β2-integrin molecules by the M1-fibrinogen network leads to neutrophil activation. To test this model, they compared heparin-binding protein release by neutrophils stimulated with M1 deletion constructs that retained the ability to bind fibrinogen but did not produce networks. Wildtype
M1 protein, but not the deletion constructs, stimulated release of heparin-binding protein from neutrophils. Consistent with this, fibrinogen fragment D, which does not support network formation due to lack of D-E-D symmetry, inhibited M1-mediated neutrophil activation.
In Brief
Macheboeuf et al. have demonstrated that neutrophil activation associated with M1 protein, a major streptococcal virulence factor, is due to the organization of fibrinogen by M1 protein into a network. The M1-fibrinogen network produces a high density of integrinbinding sites, which they propose leads to integrin clustering and neutrophil activation. Based on these results, they propose that the M1-fibrinogen interaction is a potential therapeutic target for the treatment of streptococcal toxic-shock syndrome.
References
Competing Interests
Dr. Lollar indicated no relevant conflicts of interest.