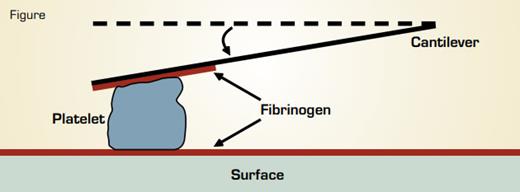
Measurement of Single Platelet Contractile Forces by Atomic Force Microscopy. A fibrinogen-coated cantilever is pressed against an activated platelet just as it lands on a fibrinogen-coated surface. Contraction of the platelet pulls the cantilever down a measureable distance toward the surface. The stiffness of the cantilever, i.e., the force required to move it a given distance, is known. Thus, the force exerted by the platelet can be calculated.
Measurement of Single Platelet Contractile Forces by Atomic Force Microscopy. A fibrinogen-coated cantilever is pressed against an activated platelet just as it lands on a fibrinogen-coated surface. Contraction of the platelet pulls the cantilever down a measureable distance toward the surface. The stiffness of the cantilever, i.e., the force required to move it a given distance, is known. Thus, the force exerted by the platelet can be calculated.
The phenomenon of clot retraction has been a favorite subject of hematologists since it was first described by William Hewson in 1780.1,2 We now know that clot retraction is due to the actomyosin-dependent contractile force of platelets that are bound to a fibrin mesh. Clot retraction alters clot organization and stiffness, which are abnormal in certain pathological states such as premature coronary disease. Dr. Lam et al. working in the laboratory of Dr. Daniel Fletcher at Berkeley used atomic force microscopy (AFM) to measure the forces produced by single, thrombin-activated platelets bound to fibrinogen (see Figure). In this method a suspension of activated platelets is viewed under a conventional optical microscope and a fibrinogen-coated surface is shifted to trap a single platelet between the surface and a fibrinogen-coated AFM cantilever. Upon contact with fibrinogen, the platelet immediately begins to contract, the cantilever is pulled down, and the distance it moves is measured optically. The cantilever is then calibrated using displacements resulting from known applied forces. Thus, the displacement force caused by single platelet contraction can be calculated.
Measurements were made using cantilevers of varying stiffness, which has the physiological correlate that clot stiffness increases with fibrinogen density. Also, by moving the surface during platelet contraction to keep its distance from the cantilever constant, it was possible to measure platelet contractile force at infinite stiffness, corresponding to isometric contraction. The authors found that as cantilever stiffness increased, platelets exhibited higher contraction forces and rates of contraction. The average contractile force of a single platelet under conditions of maximum contraction was 19 nanonewtons (nN). Because a single myosin II molecule produces a maximum force of approximately 6 pN and there are approximately 12,000 myosin II molecules per platelet, the authors estimated that the maximum contractile force of a platelet is 72 nN. Thus, the mean contractile force of a single platelet is approximately 25 percent of this maximum value. This value is similar to the efficiency of force production observed in skeletal muscle cells, which, unlike platelets, have highly ordered sarcomeres. Additionally, the force per unit area exerted by a single platelet was estimated to be more than one order of magnitude higher than produced by a single myoblast. Thus, the contractile forces generated by platelets are surprisingly high.
The authors also investigated the mechanical properties of single contracted platelets. After platelet contraction was complete, the cantilever was pulled down until adhesion to the surface or the cantilever was ruptured, which defined the adhesion strength. During the initial part of this maneuver, the ratio of force per unit area on the platelet (stress) to its relative change in length (strain) defines platelet elasticity, which is a measure of its stiffness. The authors found that the mean platelet adhesion force, 69 nN, corresponds to a force per area of more than 600 times that measured between single leukocytes and endothelial cells. Both platelet elasticity and adhesion strength were correlated with the maximum contraction force, indicating that the more force exerted by a platelet, the stiffer and more adhesive it becomes. The elasticity measurements also revealed that platelets are two orders of magnitude stiffer than platelet-free fibrin clots, suggesting that platelets reinforce the mechanical properties of the clot. The authors speculate that this may allow the platelet to act as a cross-linking center, restricting local deformation and leading to more uniform contraction of the clot.
In Brief
The results of this study provide important new insights into platelet dynamics and the properties of blood clots. The novel experimental system developed in this study could lead to probes of platelet function in health and disease.
References
Competing Interests
Dr. Lollar indicated no relevant conflicts of interest.