Associate Professor of Medicine, Duke Adult Stem Cell Transplant Program, Duke University Medical Center
Dr. Horwitz indicated no relevant conflicts of interest.
The first successful allogeneic stem cell transplantation (SCT) for sickle cell disease (SCD) was reported in 1984, establishing the procedure as the only curative option for the disease.1 However, widespread adoption of this treatment modality has been slow to evolve. There are multiple reasons for this, most important of which are the complex risk-versus-benefit considerations.
The risks of allogeneic SCT are well known, yet important advances in the field during the past decade have helped mitigate these risks. The ability to risk-stratify SCD patients has also improved recently. Patients with SCD are characterized as high-risk if they have central nervous system pathology (clinical or subclinical stroke, seizures), recurrent severe acute chest syndrome, chronic unremitting pain, or early evidence of end organ damage such as pulmonary hypertension. The appropriateness of SCT can be more firmly established in the presence of these high-risk features.
When taken together, these advances have helped to clarify the role of allogeneic SCT in the management of SCD. But despite this, the procedure remains under-utilized and the pace of development is uneven among both adult and pediatric populations.
Allogeneic SCT for Pediatric Patients with SCD
The published results of allogeneic SCT for pediatric patients with SCD are excellent. The four largest series reported in the literature describe outcomes of more than 250 children, all with a median age of less than 10 years.2-5 With rare exception, a high-risk population of patients was targeted and the donors were HLA-identical siblings. All patients received fully myeloablative bone marrow conditioning. Graft rejection ranged between 7 and 18 percent, which is higher than what might be expected from a comparable group of patients transplanted for hematologic malignancy. However, overall survival was an impressive 93 to 100 percent. These studies established the fact that successful allogeneic SCT not only eliminates the sickle-cell-induced vaso-occlusive symptomatology, but also leads to reversal of some of the end organ damage that occurred prior to the procedure.
Use of a non-myeloablative conditioning regimen prior to allogeneic SCT reduces treatment-related toxicity, so this approach holds great appeal for the treatment of patients with non-malignant disorders. Yet efforts to employ non-myeloablative conditioning for transplantation of pediatric patients with SCD have been largely unsuccessful due to high rates of graft rejection.6,7 Thus, current opinion is that children with high-risk SCD and a suitably matched donor should be offered allogeneic SCT using a conventional myeloablative conditioning regimen.
Allogeneic SCT for Adult Patients with SCD
The risk-versus-benefit considerations surrounding adult patients with SCD are, in many ways, more complex than those in pediatric patients. The risks of the disease become quite apparent in many severely affected adults, and the toll on quality of life is often more dramatic. Yet for these very same reasons, risks of the SCT are amplified. Recent efforts have focused on reducing the risks of the transplant procedure by developing the non-myeloablative approach, and while published reports are few in number and small in size, they appear to demonstrate feasibility. Dr. Biernacki and colleagues recently reviewed published reports on the use of non-myeloablative SCT for SCD.8 Twenty-four patients were identified. With a median follow-up of 2.5 years, the overall and disease-free survival was 95 percent and 85 percent, respectively. While clearly compromised by reporting bias, the study demonstrates considerably more promise for this approach in adult patients compared with that in children. A multicenter phase II protocol of non-myeloablative SCT for adult SCD is in development and expected to begin accrual in 2011.
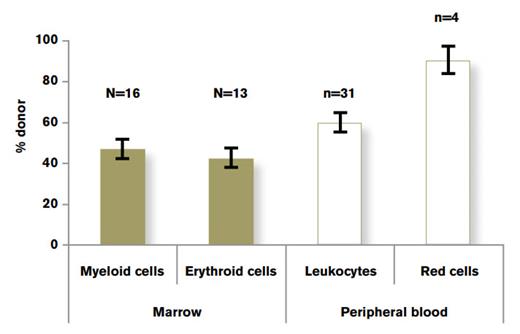
Comparison of Lineage-Specific Chimerism in Recipients of Allogeneic SCT in Patients With Hemoglobinopathy. Data drawn from three separate studies.9-11 Figure adapted from Hsieh et al.12Figure republished from Hsieh NM, Wu CJ, Tisdale JF. In mixed hematopoietic chimerism, the donor red cells win. Haematologica. 2011;96:13-15.
Comparison of Lineage-Specific Chimerism in Recipients of Allogeneic SCT in Patients With Hemoglobinopathy. Data drawn from three separate studies.9-11 Figure adapted from Hsieh et al.12Figure republished from Hsieh NM, Wu CJ, Tisdale JF. In mixed hematopoietic chimerism, the donor red cells win. Haematologica. 2011;96:13-15.
Mixed chimerism (presence of both donor and recipient hematopoiesis) is a frequently observed outcome of non-myeloablative SCT. Immunologic tolerance that characterizes mixed chimerism has been associated with a reduced frequency of severe graft-versus-host disease, but in recipients with underlying malignancies, it has also been associated with an increased risk for tumor recurrence. Donor lymphoid and myeloid chimerism is often discordant due to the significantly different lifespan of the cells in the lineage. In contrast, myeloid and erythroid cells share a relatively short lifespan, resulting in concordant chimerism. Furthermore, these lineages reflect the degree of donor stem cell engraftment. However, hemoglobinopathy transplant recipients with mixed chimerism typically display greater than 90 percent donor peripheral blood red cell chimerism, irrespective of the degree of donor leukocyte chimerism (Figure).9-11
The tremendous survival advantage afforded to normal erythroid cells is a consequence of ineffective host erythropoiesis, and there are significant clinical implications of this phenomenon. First, it provides a strong rationale for further development of a reliable nonmyeloablative SCT approach that fosters stable mixed chimerism. In addition, it suggests a promising role for gene therapy in SCD patients for whom high-level gene correction of stem cells is difficult to obtain, but might not be necessary for therapeutic benefit.
Current Challenges and Future Directions
Access remains the major limitation to the broader use of SCT for treatment of severely affected patients with SCD, due to lack of private and government-funded insurance coverage. However, powerful pharmacoeconomic data demonstrating the advantage of early, definitive therapy for SCD versus lifelong supportive care may help improve coverage for SCT. In addition, the rarity of both unaffected HLA-identical sibling and matched unrelated donors severely limits accessibility of SCT therapy. Ongoing efforts to improve outcomes of umbilical cord blood and haploidentical transplantation for SCD may one day address this problem as well.