To most, the complement system resides in one of the dark corners of hematology, occasionally dimly illuminated when the direct antibody test is reported as “positive” for C3 in a patient with autoimmune hemolytic anemia. Undeniably, the complement system, with its three overlapping pathways (classical, alternative, and lectin), is complex, but the development of new and emerging targeted therapies for complement-mediated diseases necessitates understanding of this arcane system. The purpose of this article is to shine a light into that shadowy corner of hematology in which complement resides by reviewing three diseases (atypical hemolytic uremic syndrome [aHUS], paroxysmal nocturnal hemoglobinuria [PNH], and hereditary angioedema [HAE]) in which inherited or acquired mutations that affect complement system proteins underlie or contribute to disease pathophysiology. A comprehensive review of the biochemistry and pathobiology of the complement system is beyond the scope of this report; however, an excellent one can be found in the first reference.1
aHUS
Hematologists encounter patients with aHUS when they are asked, along with their nephrology colleagues, to evaluate a patient with the triad of severe thrombocytopenia, microangiopathic hemolytic anemia, and renal failure. The clinical features suggest a thrombotic microangiopathic process, and the most common etiology is hemolytic-uremic syndrome (HUS).2 Approximately 90 percent of HUS is caused by infection with shiga toxin and shiga-like toxin (verotoxin)-producing bacteria, particularly enterohemorrhagic E. coli. Less commonly, invasive Streptococcus pneumoniae underlie the thrombotic microangiopathy. With supportive care, patients who develop typical diarrhea-positive HUS have a favorable prognosis, with spontaneous resolution of the classic disease triad in approximately 90 percent of cases (although temporary dialysis may be required). For patients who present with the diarrhea-negative form of the disease (aHUS), however, the prognosis is bleak with 50 percent developing end-stage renal failure and 25 percent dying from complications of the disease.
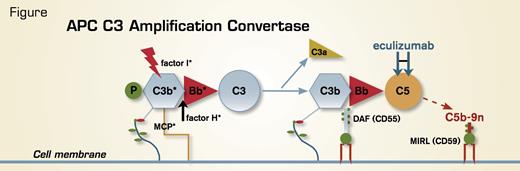
Recently, new insights into the etiology of aHUS have emerged, with studies by a number of investigators demonstrating that aberrant regulation of the amplification C3 convertase of the alternative pathway of complement (APC) predisposes to disease acquisition (Figure). Molecularly defined abnormalities have been identified in approximately half of the cases. Specifically, complement factor H is mutant in approximately 30 percent of cases, membrane cofactor protein (MCP, CD46) in approximately 10 percent, and complement factor I in approximately 10 percent. In another 10 percent, auto-antibodies against factor H have been identified. Disease penetrance, however, is variable, as risk polymorphisms may be required for development of aHUS. New DNA sequencing technology will likely solve the current problems of both time delay and expense required to identify the genetic basis of the disease in an individual patient. Moreover, these techniques will reveal novel genetic mechanisms that underlie the pathobiology in the 50 percent of patients who do not have mutations in APC C3 amplification convertase components.
The APC. Unlike the classical pathway of complement that requires specific antibody-antigen binding to initiate activation, the APC is in a state of continuous activation (termed “tick-over”). Covalently bound C3b serves as the nidus for formation of the amplification C3 convertase of the APC, consisting of C3b, activated factor B (Bb) (the enzymatic subunit of the complex), and factor P (properdin, a stabilizing protein). The C3 convertase amplifies the APC by cleaving many molecules of C3 to C3b (with release into the fluid phase of the C3a anaphylatoxin). These activated C3b molecules form additional C3 convertases and also C5 convertases (C3bBbC3bP) that lead eventually to generation of the cytolytic membrane attack complex (C5b-9n). Because the APC is primed for attack continuously, elaborate mechanisms for self-recognition and for protection of the host against APC-mediated injury have evolved. Both fluid-phase (factor H and factor I) and membranebound proteins (MCP, DAF, and MIRL) are involved in this process. APC regulatory proteins that have been shown to be mutant in patients with aHUS are indicated with an asterisk. The GPI-AP regulatory proteins, DAF (CD55) and MIRL (CD59), are deficient in PNH, accounting for the complement-mediated hemolysis characteristic of the disease.
The APC. Unlike the classical pathway of complement that requires specific antibody-antigen binding to initiate activation, the APC is in a state of continuous activation (termed “tick-over”). Covalently bound C3b serves as the nidus for formation of the amplification C3 convertase of the APC, consisting of C3b, activated factor B (Bb) (the enzymatic subunit of the complex), and factor P (properdin, a stabilizing protein). The C3 convertase amplifies the APC by cleaving many molecules of C3 to C3b (with release into the fluid phase of the C3a anaphylatoxin). These activated C3b molecules form additional C3 convertases and also C5 convertases (C3bBbC3bP) that lead eventually to generation of the cytolytic membrane attack complex (C5b-9n). Because the APC is primed for attack continuously, elaborate mechanisms for self-recognition and for protection of the host against APC-mediated injury have evolved. Both fluid-phase (factor H and factor I) and membranebound proteins (MCP, DAF, and MIRL) are involved in this process. APC regulatory proteins that have been shown to be mutant in patients with aHUS are indicated with an asterisk. The GPI-AP regulatory proteins, DAF (CD55) and MIRL (CD59), are deficient in PNH, accounting for the complement-mediated hemolysis characteristic of the disease.
Understanding the molecular basis of aHUS has important prognostic and therapeutic implications, as disease penetrance, response to plasma exchange, immunosuppressive therapy, and kidney transplant are influenced by the mutational status of the patient.2 Plasma exchange appears to be more efficacious in patients with mutant factor H than in those with mutant factor I, whereas 80 to 90 percent of patients with mutant MCP remit spontaneously without plasma exchange. Long-term dialysis-free survival has been observed in 60 to 70 percent of patients with auto-antibodies to factor H who are treated with a combination of plasma exchange, immunosuppressant drugs, and rituximab. Anecdotal reports of responses to the complement inhibitory drug, eculizumab, led to initiation of two multicenter, open-label clinical studies for both adult (NCT01194973) and pediatric patients (NCT01193348). Except for patients with mutation in MCP (a cell surface protein, Figure), kidney transplant for patients with aHUS is unsatisfactory because the other APC C3 amplification convertase components are plasma proteins that are produced in the liver. And while kidney/liver transplantation is feasible, procedure-related morbidity and mortality are daunting.
Table. C1 Inhibitor - Master Serine Protease Inhibitor
| Targets of C1 Inhibitor . | ||
|---|---|---|
| Coagulation Cascade | Classical and Lectin Pathways of Complement | Constant Cascade |
| Factor XIIa, Factor XIa | C1rC1s*, MASP-2† | Kallikrein§ |
| Targets of C1 Inhibitor . | ||
|---|---|---|
| Coagulation Cascade | Classical and Lectin Pathways of Complement | Constant Cascade |
| Factor XIIa, Factor XIa | C1rC1s*, MASP-2† | Kallikrein§ |
*C1r and C1s are the serine protease components of the trimolecular first component of the classical pathway of complement that also includes C1q (the immunoglobulin binding component of the complex). C1r and C1s activate C2 and C4 of the classical pathway.
†Mannose-binding lectin-associated serine protease-2. Like C1r and C1s, MASP-2 activates C2 and C4.
§Kallikrein is the serine protease that converts high-molecular-weight kininogen to bradykinin (the protein that binds to bradykinin type-2 receptors and induces endothelial cell activation). Ecallantide (ref. 11) and icatibant (ref. 10) are two therapeutic options for treating HAE. Ecallantide substitutes for C1 inhibitor by blocking kallikrein while icatibant acts downstream by inhibiting the binding of bradykinin to its receptor.
PNH
PNH has a special place in hematology and complementology, as identification of the molecular basis of the intravascular hemolysis that is the clinical hallmark of the disease led to a remarkable number of discoveries that helped identify and characterize the APC and define the physiology and pathophysiology of the complement system in humans.3 The discoveries began with the seminal observations of Thomas Hale Ham in the late 1930s and led ultimately to the development of the first successful targeted therapy for a complement- mediated disease when eculizumab was approved for treatment of PNH in 2007.4
PNH is an acquired disorder that arises as a consequence of somatic mutation in one or more hematopoietic stem cells of PIGA, a gene located on the X chromosome that is required for synthesis of the glycosylphosphatidylinositol (GPI) moiety that anchors some proteins to the cell surface (Figure). Consequently, all GPI-anchored proteins (GPI-APs) are deficient on the mutant hematopoietic stem cells and their progeny. The complement-mediated intravascular hemolytic anemia and the resulting hemoglobinuria that are the clinical hallmarks of PNH are a consequence of deficiency of the GPI-anchored complement regulatory proteins, CD55 (decay accelerating factor) and CD59 (membrane inhibitor of reactive lysis). The intravascular hemolysis of PNH can be controlled with eculizumab, a humanized monoclonal antibody that disrupts formation of the cytolytic membrane attack complex of complement by binding to the fifth component of complement (C5) (Figure). PNH has been the subject of recent comprehensive reviews published in The Hematologist5 and Blood.6,7
HAE
Unlike aHUS and PNH, the defining clinical features (episodic, nonurticarial, nonpuritic subcutaneous, and submucosal swelling affecting primarily the upper respiratory and gastrointestinal systems) of HAE are non-hematologic. As a result, hematologists are usually not involved in the initial diagnosis of the disease. HAE displays an autosomal dominant inheritance pattern and is due to mutation of C1 inhibitor, a serine protease inhibitor that binds irreversibly to target enzymes of the classical and lectin pathways of complement, the intrinsic pathway of coagulation, and the contact pathway involved in endothelial cell activation (Table).8 Once suspected, based on recurrent angioedema without urticaria, recurrent abdominal pain and vomiting, laryngeal edema, and a positive family history, the diagnosis can be made by measuring plasma concentrations of C1 inhibitor and complement C4. A number of treatment options are available, and these have been the subject of four recent publications in The New England Journal of Medicine.8-11 Notably, two of the inhibitors (ecallantide and icatibant) specifically target the contact cascade, with no direct effect on either the complement or coagulation cascade, indicating that disease pathology is primarily the result of endothelial cell activation rather than a consequence of deregulation of the coagulation or complement cascades (Table).