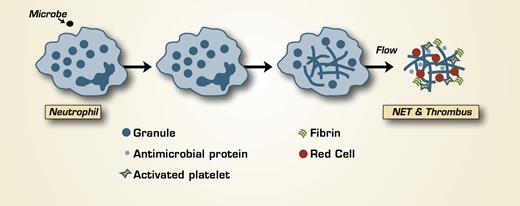
The anti-microbial defense provided by neutrophils includes phagocytosis of microbes into phagolysosomes and degranulation with release of anti-microbial molecules at the site of infection. Recently, an additional defense mechanism was discovered — the formation of neutrophil extracellular traps (NETs), which are produced following the release of the nuclear contents of the neutrophil into the extracellular space.1 NETs have been identified in all vertebrate lineages and function in innate immunity. They are composed of chromatin components, including histones, and neutrophil antimicrobial proteins. Microbes are trapped in NETs, where they encounter high concentrations of anti-microbial proteins.
Both activation of the hemostatic mechanism and inflammation are vertebrate responses to infection, and there has been an increasing awareness of crosstalk between these two systems.2 Now, an association between hemostasis and NETs has been identified by Fuchs et al. in the laboratory of Denisa Wagner at Harvard Medical School. The authors produced NETs in vitro and studied their interaction with whole blood or platelet-rich plasma under conditions of flow at both arterial and venous shear rates. NETs aligned in the direction of flow; platelets adhered to them rapidly and aggregated. Additionally, red cells adhered to NETs. NETs dissolved in the presence of heparin, which, as a negatively charged sulfated polysaccharide, is known to bind positively charged histones. Interestingly, histones H3 and H4 directly produced platelet aggregation, which was blocked by heparin. Fibronectin, von Willebrand factor (VWF), and fibrinogen bound to NETs, and the presence of fibrinogen resulted in thrombin-dependent fibrin deposition. NETs also were studied in a baboon model of deep-venous thrombosis produced by catheter occlusion of the iliac vein. Thrombus formation resulted in elevated plasma levels of DNA with kinetics that mimicked the elevation of fibrin(ogen) D-dimer that occurs in this model. Post-mortem analysis of the injured vein revealed nuclear DNA and histones in the thrombus as well as VWF.
In Brief
The results of this study indicate that the association of NETs with fibrin may serve to organize the structure of the thrombus and influence platelet activation (in part through histones acting as agonists), fibrin formation, and fibrinolysis. At a site of infection, the anti-microbial and prohemostatic functions of NETs may function to prevent or limit sepsis by local containment and killing. However, extracellular histones may contribute to endothelial dysfunction and exacerbate the pathological sequelae of sepsis.3 The beneficial and pathological effects of the linkage between NETs and the hemostatic system remain to be established.