The discovery of the adverse effects of erythropoiesis-stimulating agents (ESAs) on mortality and/or tumor progression in eight clinical trials and recent meta-analyses has led to substantial revisions in the guidelines for ESA therapy in chemotherapy-induced anemia.1 Furthermore, the United States Food and Drug Administration has installed a Risk Evaluation and Mitigation Strategy to ensure the safe use of ESAs. This program mandates that prescribers of ESAs must participate in the “Assisting Providers and Cancer Patients with Risk Information for the Safe use of ESAs” (APPRISE) program, provide patients with a medication guide discussing the benefits and risks of ESAs, and obtain written informed consent upon each new course of treatment. Yet beyond venous thromboembolism, which is a well-recognized risk of ESA therapy, large gaps persist in our understanding of the mechanisms by which ESAs can promote deleterious effects in cancer patients. At issue is whether expression levels of erythropoietin receptor (EpoR) on the surface of tumor cells or tumor blood vessels are sufficient to transduce signals that impart significant biologic effects in response to Epo stimulation.
Using a novel, specific, and sensitive monoclonal anti-EpoR antibody and a semi-quantitative western immunoblot assay, scientists at Amgen recently reported that total EpoR expression in a panel of 66 human tumor cell lines ranged from undetectable (28 lines) to as high as 1,600 to 3,200 EpoR dimers per cell (four lines), corresponding to 8.3 percent to 20 percent of the EpoR level in erythroid progenitor cells.2 Using an alternative specific monoclonal anti-EpoR antibody, our group similarly found that cell surface EpoR levels in a panel of 29 human tumor cell lines ranged from 1.2 percent to 25.2 percent (mean 8.3%) of the EpoR level in Epo-dependent UT7EPO cells.3 Thus, EpoR is clearly present at significant levels in at least certain cancer cell lines. However, disparities exist among reports from different laboratories regarding the ability of Epo to stimulate signal transduction and impart cellular responses,4 in some cases even among the same cell lines,2,5,6,7 and a clear threshold level of expression at which EpoR is functional has not been defined. Moreover, it has been difficult to establish animal models that recapitulate the clinically observed adverse effects of exogenous ESAs.4,8
The discrepancies and lack of consensus from pre-clinical studies in cell lines and animal models highlights the notion that definitive confirmation or denial of a significant role for tumor-expressed EpoR must come from clinically focused studies using primary tumor samples. Correlating tumor EpoR levels with tumor progression and survival outcomes in completed and ongoing clinical trials of ESAs offers a direct means of testing the EpoR-tumor hypothesis. For hematopoietic cancers, surface EpoR levels can be measured specifically by flow cytometry. For example, we recently discovered that EpoR levels on the surface of abnormal bone marrow plasma cells vary considerably among patients with multiple myeloma, whereas the corresponding lymphocytes from these patients were uniformly negative.9
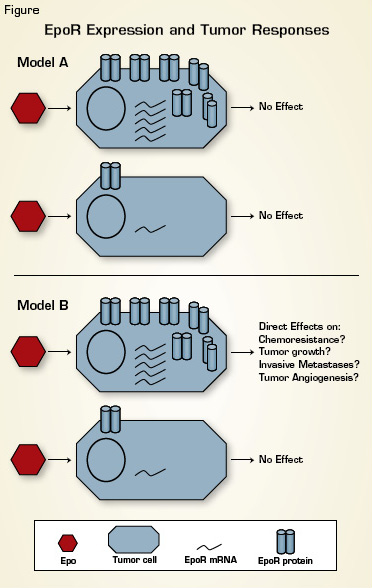
Tumor EpoR Expression and Clinical Outcomes. Recent studies have established that EpoR mRNA and protein levels vary substantially among tumor samples from different patients. Model A: EpoR levels are not sufficient to bestow Epo responsiveness. Model B: Adverse effects of Epo in cancer patients are mediated via direct effects on tumor cells in a manner that depends upon tumor EpoR levels. Clinically focused studies using primary patient tumor specimens are needed to discriminate between these two models.
Tumor EpoR Expression and Clinical Outcomes. Recent studies have established that EpoR mRNA and protein levels vary substantially among tumor samples from different patients. Model A: EpoR levels are not sufficient to bestow Epo responsiveness. Model B: Adverse effects of Epo in cancer patients are mediated via direct effects on tumor cells in a manner that depends upon tumor EpoR levels. Clinically focused studies using primary patient tumor specimens are needed to discriminate between these two models.
Measurements of EpoR levels in solid tumors are confounded by the lack of anti-EpoR antibodies that are sufficiently sensitive and specific for definitively detecting EpoR by immunohistochemistry.10,11 Even the most sensitive antibody to date (Amgen A82) was reported to exhibit some non-specific staining in negative control cells in this application.12 EpoR mRNA represents a surrogate but must be tempered against technical challenges relating to the extensive degradation that characterizes RNA extracted from formalin-fixed archival tumors, concerns as to whether EpoR mRNA uniformly correlates with surface protein levels, and the potentially confounding effects of cell-type heterogeneity in tumor samples. Nevertheless, we recently optimized a sensitive and specific quantitative RT-PCR assay for EpoR mRNA in archival tumors and found that EpoR mRNA levels vary as much as 30-fold among breast cancer and head and neck cancer specimens.3 Moreover, laser capture microdissection studies in a panel of breast cancers revealed that variation of EpoR mRNA levels was greater among inter-tumor specimens than intra-tumor specimens (i.e., between tumor epithelial and endothelial cell fractions) (CPM, unpublished observation). Thus, despite the known heterogeneity in tumor vasculature, solid tumors can be segregated by their overall EpoR levels.
Ultimately, future investigation in this area must determine whether there is clinical utility in measuring EpoR protein and/or EpoR mRNA levels in tumor specimens prior to initiation of ESA therapy and whether the Epo/EpoR axis represents a target for cancer therapy.13 Well-designed clinical studies that incorporate corollary analyses of EpoR levels along with clinical outcomes data will be helpful to further our understanding of this important issue.
Acknowledgments The authors would like to thank Drs. C. Anthony Blau and Pam Becker for insightful discussions. Dr. Miller is supported by a mentored research scholar grant from the American Cancer Society [117682-MRSG-09-268-01-CCE]. Dr. Burwick is supported by a T32 training grant in hematology from the National Institutes of Health.