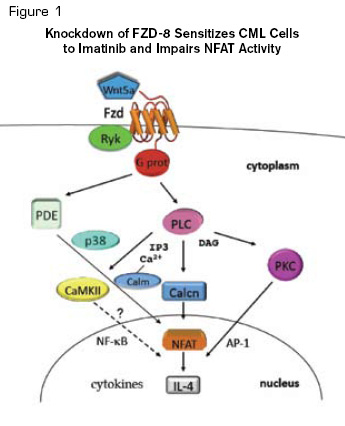
Knockdown of FZD-8 Sensitizes CML Cells to Imatinib and Impairs NFAT Activity. Diagrammatic representation of the Wnt/Ca2+/NFAT pathway. Pathway details are described in the text. Reprinted from Cancer Cell, Volume 18 /Issue 1, Gregory MA, Phang TL, Neviani P, et al. Wnt/Ca2+/NFAT signaling maintains survival of Ph+ leukemia cells upon inhibition of Bcr-Abl, 74-87, Copyright (2010), with permission from Elsevier.
Knockdown of FZD-8 Sensitizes CML Cells to Imatinib and Impairs NFAT Activity. Diagrammatic representation of the Wnt/Ca2+/NFAT pathway. Pathway details are described in the text. Reprinted from Cancer Cell, Volume 18 /Issue 1, Gregory MA, Phang TL, Neviani P, et al. Wnt/Ca2+/NFAT signaling maintains survival of Ph+ leukemia cells upon inhibition of Bcr-Abl, 74-87, Copyright (2010), with permission from Elsevier.
Wnt proteins are extracellular ligands that activate a variety of signaling pathways important in cellular functions, including proliferation and differentiation. The so-called “canonical” pathway involves their binding to cell surface co-receptors from the LRP and Frizzled families, which then allow β-catenin proteins to enter the nucleus, where they associate with the transcription co-activator Lef-Tcf to activate specific genetic programs. Experimental evidence suggests that aberrant Wnt/catenin activation occurs in many cancers. In CML, β-catenin activation in the granulocytemacrophage progenitor (GMP) fraction was seen in samples from blastcrisis patients. Furthermore, alternative splicing of mRNA for an important regulator of the β-catenin pathway, GSK3β, was shown to occur with progression-facilitated β-catenin activation. Moreover, gene expression studies have demonstrated activation of the Wnt/catenin pathway, including aberrant expression of frizzled family members, in CML progression. Several pharmaceutical companies have been working on targeting this pathway, either directly or by influencing activity of pathways with crosstalk to the Wnt/catenin pathway (e.g., Hedgehog).
Wnt also signals through “non-canonical” pathways, including Ca2+/NFAT. In this pathway, Wnt-mediated dephosphorylation of NFAT by calcineurin causes NFAT to translocate to the nucleus, where it promotes transcription of various cytokine genes (Figure 1). While the role of the pathway has been unclear in cancer, the recent work by Gregory et al. suggests it may be involved in the survival of Bcr-Abl leukemia despite TKI therapy.
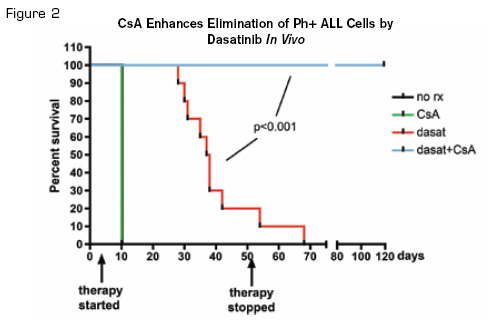
CsA Enhances Elimination of Ph+ ALL Cells by Dasatinib In Vivo. Kaplan-Meier curve showing survival of mice receiving the indicated therapy. Mice were sacrificed when moribund, and all showed clear evidence of leukemia in blood, bone marrow, and spleen. Reprinted from Cancer Cell, Volume 18 /Issue 1, Gregory MA, Phang TL, Neviani P, et al. Wnt/Ca2+/NFAT signaling maintains survival of Ph+ leukemia cells upon inhibition of Bcr-Abl, 74-87, Copyright (2010), with permission from Elsevier.
CsA Enhances Elimination of Ph+ ALL Cells by Dasatinib In Vivo. Kaplan-Meier curve showing survival of mice receiving the indicated therapy. Mice were sacrificed when moribund, and all showed clear evidence of leukemia in blood, bone marrow, and spleen. Reprinted from Cancer Cell, Volume 18 /Issue 1, Gregory MA, Phang TL, Neviani P, et al. Wnt/Ca2+/NFAT signaling maintains survival of Ph+ leukemia cells upon inhibition of Bcr-Abl, 74-87, Copyright (2010), with permission from Elsevier.
The authors used an RNAi-based “synthetic lethal” screen to identify pathways whose inhibition sensitized CML cells to TKIs. A large shRNA library was transduced into CML cell lines, and shRNAs associated with resistance to imatinib were identified. Among these were the Wnt receptor Frizzled-8 (FZD-8) as well as components of the Ca2+/NFAT pathway. Transduction of shRNA targeting FZD-8 verified that inhibition sensitized CML cells to imatinib and that this caused a decrease in NFAT activation. The authors next targeted the control of NFAT by calcineurin and demonstrated that the calcineurin inhibitor cyclosporin A (CsA) inhibited NFAT activation. They then used a Ph+ ALL mouse model to demonstrate that treatment with the TKI dasatinib and CsA was associated with a dramatic survival advantage compared with either drug alone (Figure 2). The data thus suggest a role of the Wnt/Ca2+/NFAT pathway in TKI resistance and suggest a novel method to induce sensitivity to resistant Bcr-Abl leukemia.
Curiously, a variation of this mouse model may have already been done in humans. Several transplant groups have given TKI to patients who have undergone allogeneic transplant for advanced-phase CML or Ph+ ALL. Preliminary data suggest this is very effective in preventing relapse. Since most of these patients are already on a calcineurin inhibitor, one wonders if the efficacy is not just from blocking Bcr-Abl activity but promoting apoptosis by blocking the Wnt/Ca2+/NFAT pathway.
In Brief
Treatment of advanced-phase CML is poor. Even transplantation in frank blast crisis cures only 10 to 20 percent of cases. However, prognosis with transplantation is greatly improved if patients achieve a remission prior to transplant. The ability to use a TKI and a calcineurin inhibitor (e.g., dasatinib and cyclosporin) to treat patients would be a remarkable advance to stabilize and “debulk” patients while preparing for allogeneic transplant.
Competing Interests
Dr. Radich indicated no relevant conflicts of interest.