MicroRNAs (miRNAs) are small non-coding RNAs of about 18 to 24 nucleotides in length that regulate gene expression and thus influence such processes as development, differentiation, proliferation, and apoptosis.1 MiRNAs exert their biological effects by binding in a sequence-specific manner for the most part to the 3’ untranslated region (3’ UTR) of the target mRNA, causing protein translation inhibition and/or mRNA degradation. It has been estimated that miRNAs regulate about 30 percent of human genes.1 Findings over the past eight years strongly support a role for deregulated miRNA activity in the initiation and progression of cancer, including hematologic malignancies (HM).1 We will briefly summarize the state of the field and discuss future directions.
Expression-profiling studies in disease classification/diagnosis
Following our initial demonstration of deletion/down-regulation of miR-15a/miR-16-1 in B cells of patients with chronic lymphocytic leukemia (CLL), additional studies established that malignant hematopoietic cells exhibited distinctive miRNA expression signatures compared to their normal counterparts. The advent of high-throughput miRNA profiling established that such miRNA expression signatures allowed discrimination with high accuracy among different cytogenetic and molecular subtypes of leukemias/lymphomas and multiple myelomas.2 Although there were similarities in miRNA signatures among the disease-specific miRNA profiling studies published in the literature, substantial differences were also noted. This could be explained by the use of different platforms to interrogate miRNA expression, heterogeneity in the sample population, or control cells used (CD34+ selected vs. total bone marrow).2 Novel platforms are now increasingly available, including next generation sequencing, which may improve sensitivity and accuracy of miRNA detection and, in addition, may enable discovery of novel miRNAs and mutations/polymorphisms. The presence of circulating miRNAs within microvesicles in the peripheral blood is intriguing; however, the functional significance remains to be fully understood.
Functional studies
Gain- and loss-of-function experiments, including animal models in combination with target prediction analyses have provided insights into the role of miRNAs in leukemogenesis. In AML, miR-29b is emerging as an important tumor suppressor.3-4 We have reported that miR-29b modulates DNA methylation by targeting DNA methyltransferases (DNMT)-1, 3A, and 3B. Restoring miR-29b expression in AML cell and primary samples resulted in global DNA hypomethylation and gene re-expression of methylated and silenced tumor suppressor genes.3 Furthermore, miR-29b overexpression suppresses cell proliferation and induces cell death by directly targeting the cell-cycle regulator CDK6 and the anti-apoptotic MCL-1. The antitumor effects were validated in murine xenograft models.4
Recently, we established the central role of miR-29b in a transcriptional network that regulates KIT transcription in AML cells.5 Loss of miR-29b unblocks expression of the transcription factor Sp1 that in combination with NF-κB binds to the KIT promoter and activates its transcription. We further showed that KIT overexpression is prognostic in AML and is involved in leukemogenesis promoting cell proliferation.5 In CML, our group identified that upon disease progression to blast crisis there is loss of miR-328, which directly interferes with the activity of hnRNP-E2, a poly(rC)-binding protein that suppresses neutrophil maturation in blast-crisis CML through translational inhibition of C/EBPα expression.6 In contrast to the classical miRNA effector pathways, the C-rich mature miR-328 interacts in a non-sequence-specific manner with hnRNP-E2 and prevents its binding to the CEBPA intercistronic region (decoy activity), thus allowing C/EBPα expression, which directly enhances miR-328 transcription.6
Lastly, miR-15-a/miR-16-1, which is lost in CLL patients by genomic deletion and mutations, directly targets BCL-2, a known anti-apoptotic gene that is up-regulated in a subset of CLL patients. A negative correlation was found between miR-15-a/miR-16-1 and Bcl-2 protein expression in CLL patients, and ectopic overexpression resulted in apoptosis in leukemic cells. It was recently reported that miR-15-a/miR-16-1 knockout mice developed a CLL-like disease and lymphomas, further supporting a tumor suppressor role in CLL. These are some examples of functional implications of miRNAs in HM, thereby supporting a critical pathogenic role of miRNAs. The challenge for the future is to integrate miRNAs into the context of oncogenic pathways in HM and develop more animal models with gain or loss of function of miRNAs.
MiRNAs as biomarkers
In AML, two studies reported miRNA signatures associated with outcome. In high-risk, cytogenetically normal, young patients (< 60 years old), defined by the presence of FLT3-ITD mutations or wild-type nucleophosmin or the combination of both genotypes, high levels of miR-181 family members were associated with better event-free survival.7 In a different subset of AML patients (older with intermediate and poor-risk cytogenetics), high levels of miR-199a and miR-191 were associated with lower overall and disease-free survival.8 It has been reported that lower levels of miR-29b are associated with worse outcome in mantle cell lymphoma. In CLL, an miRNA signature, including high levels of miR-155 and miR-146, was associated with disease progression.9 Concerning miRNAs as predictor for treatment response, our group recently reported that higher pre-treatment levels of miR-29b were associated with achievement of clinical response to single-agent decitabine (20 mg/m2 for 10 days) in elderly AML patients.10 Future directions include standardization of assays to measure miRNAs in a reliable and reproducible way to test further their biomarker potential in clinical trials.
Targeting miRNAs in hematologic malignancy
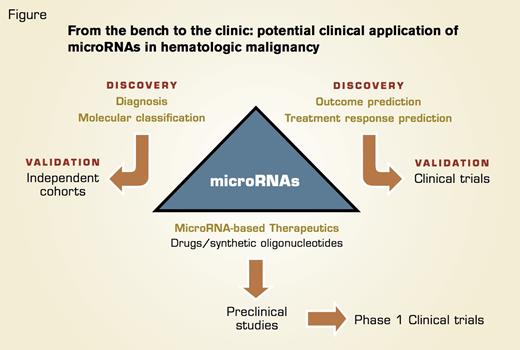
In This Schema, we Outline the Potential Clinical Applications of microRNAs in Hematologic Malignancies. Translation of molecular and prognostic biomarker discoveries to the clinic will require extensive validation in independent cohorts of patients using validated and reliable platforms for miRNA detection. These results should then be tested prospectively in clinical trials. Targeting microRNA’s expression in hematologic malignancy requires identification of the candidate microRNAs, evaluation of the different strategies to achieve expression modulation (drugs, antisense, or oligonucleotide mimics), and validation in preclinical animal models. Successful candidates then can be moved forward to phase I clinical trials for toxicity evaluation and pharmacokinetics/pharmacodynamics studies.
In This Schema, we Outline the Potential Clinical Applications of microRNAs in Hematologic Malignancies. Translation of molecular and prognostic biomarker discoveries to the clinic will require extensive validation in independent cohorts of patients using validated and reliable platforms for miRNA detection. These results should then be tested prospectively in clinical trials. Targeting microRNA’s expression in hematologic malignancy requires identification of the candidate microRNAs, evaluation of the different strategies to achieve expression modulation (drugs, antisense, or oligonucleotide mimics), and validation in preclinical animal models. Successful candidates then can be moved forward to phase I clinical trials for toxicity evaluation and pharmacokinetics/pharmacodynamics studies.
One of the most appealing properties of miRNAs as therapeutic agents is their ability to target multiples genes, frequently within the context of a network, making them very efficient in regulating distinct biological cell processes relevant to normal and malignant cell homeostasis. One can envision that there is potential to use strategies to modulate miRNA expression in specific diseases. In CLL, restoring miR-15a/miR-16-1 may block BCL-2 expression and induce apoptosis, while in AML over-expressing miR-29b may block apoptosis pathways (MCL-1), proliferation (CDK6), methylation (DNMT1, 3a, and 3b), and kinome alterations (c-KIT). These strategies may include the use of mimic or antisense oligonucleotides, drugs or small molecule compounds that affect miRNA transcription. Current challenges include delivery of synthetic oligonucleotides, stability, and safety.11
In summary, miRNA deregulation is involved in the initiation and progression of HM, and there is potential to use miRNAs to improve disease molecular classification and to establish novel biomarkers to predict outcome and treatment response and develop novel miRNA-based therapeutic strategies (Figure).
References
Competing Interests
Drs. Garzon and Croce indicated no relevant conflicts of interest.