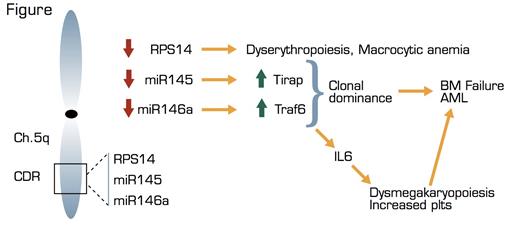
Myelodysplastic syndromes (MDS) comprise a group of clonal hematopoietic diseases characterized by cytopenias, dysplasia in one or more of the major myeloid lineages, ineffective hematopoiesis, and an increased risk of development of acute myeloid leukemia (AML). In some cases of MDS, all or part of one copy of the long arm of chromosome 5 (5q) is deleted in cells of the myelodysplastic clone. The degree of deletion within 5q can vary, and molecular studies have revealed a commonly deleted region (CDR) that is required for the 5q- syndrome, the specific findings of which include refractory anemia and normal or elevated platelet counts with dysplastic micromegakaryocytes in the bone marrow (BM). The CDR is about 1.5 million bases long and encodes many different genes. In 2008, Ebert and colleagues, then in the laboratory of Todd Golub, showed that haploinsufficiency of the RPS14 gene, which is within the CDR, alone was able to cause refractory anemia analogous to that found in the 5q- syndrome.1 In this recent manuscript from the laboratory of Aly Karsan in Vancouver, Starczynowski and colleagues show that deletion of two microRNAs, miR145 and miR146a, which also lie within the CDR, can cause megakaryocytic dysplasia with increased platelets and can lead to BM failure and AML. MicroRNAs (miRs) are small, 18- to 24-nucleotide non-coding RNAs that regulate either stability or translation into protein of partially complementary target messenger RNA molecules.
The investigators first showed that the expression levels of miR145 and miR146a are decreased in the MDS cells from patients with 5q- syndrome, whereas they are expressed normally in MDS cells with intact 5q from other patients. When the investigators knocked down expression of miR145 and miR146a in mouse BM, the mice developed many aspects of the 5q- syndrome, including dysplastic megakaryocytes and elevated platelet counts, but erythropoiesis was normal in these mice. Over time, loss of miR145 and miR146a led to loss of normal hematopoietic cells in the BM, a phenomenon referred to as clonal dominance, which led to either BM failure or AML in the mice.
Based on their RNA sequence, miR145 and miR146a potentially target more than 500 genes, and miR146a has been implicated in many diseases including ALL, AML, rheumatoid arthritis, and breast cancer.2 In order to determine the mechanism(s) by which loss of miR145 and miR146a cause the 5q- syndrome, the investigators studied two target genes of these miRs, TRAF6 and TIRAP, that have been implicated in the innate immune system. When the investigators overexpressed TRAF6 (which is not only down-regulated by miR146a, but also activated by TIRAP, which in turn is down-regulated by miR145) in mouse BM, they again produced a phenotype similar to the 5q- syndrome with evolution to either AML or BM failure. Because interleukin 6 (IL6) is known to be up-regulated by TRAF6, they tested the effects of TRAF6 overexpression in IL6 knockout mice. Although the mice did not develop abnormal megakaryocytes, they did develop AML. Thus, IL6 appears to mediate TRAF6’s effect on megakaryocytes and platelets, whereas TRAF6 alone eventually causes clonal dominance and progression to leukemia.
In Brief
Whether the puzzle of the 5q- syndrome is solved remains to be shown; these studies beautifully complement those showing that haploinsufficiency of RPS14 causes the erythroid dysplasia in MDS and suggest that haploinsufficiency of RPS14 plus miR145 and miR146a may cause all of the known manifestations of the 5q- syndrome.
References
Competing Interests
Dr. Krause indicated no relevant conflicts of interest.