Our approach to idiopathic thrombocytopenic purpura (ITP) has undergone a number of changes over the past several years. A number of these have been highlighted in three excellent reviews published in Blood in 2009. First, there is now a standard nomenclature. ITP is now immune thrombocytopenia1 ; the “i” is no longer “idiopathic” and “purpura” is no longer part of the name. Second, categorization of the underlying mechanisms of secondary ITP has been undertaken. 2 Finally, while an official ASH guideline update is in progress, an extensive guideline update has been provided by an international group of experts led by Provan et al.3
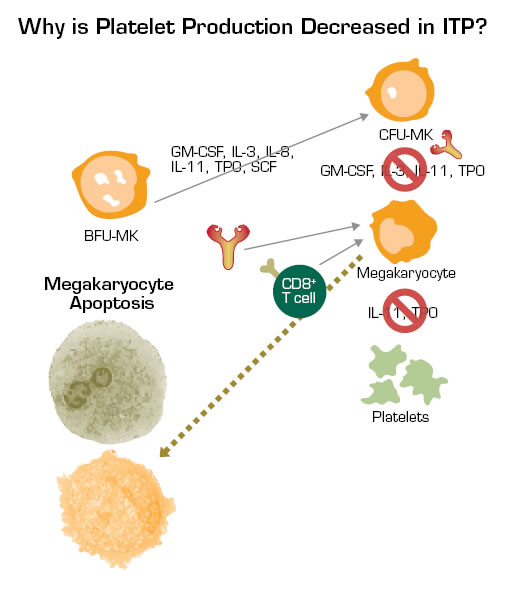
The most fundamental area of change has been in our understanding of pathophysiology. The “old” concept was that thrombocytopenia resulted from antibody-mediated platelet destruction. There are two “new” concepts; the most developed is that the same antibodies that mediate platelet destruction also mediate impaired platelet production by damaging megakaryocytes and/or blocking their ability to release proplatelets (Figure). A much less well-understood area is the basis of the 10 to 20 percent of cases that are not antibody-mediated. This number is derived from the percentage of patients who do not respond to IVIG and splenectomy. The original pioneering studies in which infusions of ITP plasma into normal subjects were shown to produce thrombocytopenia only reduced the platelet count in 16 of 26 cases. Some of the 10 non-effective plasmas were probably still from patients with antibody-mediated platelet destruction, but perhaps all or most of the antibody was bound to the platelets and not free-floating in plasma. Other cases may reflect myelodysplasia too early to be diagnosed. An exciting but to-be-explored area involves the role of platelet reactive cytotoxic CD8+ cells. These cells clearly exist, but their clinical relevance is not known. Finally, an area of intensive investigation involves T-regulatory cells, which have been reported to be deficient in ITP in several studies.
Studies Have Shown That Anti-Platelet Antibodies can Inhibit Megakaryocyte Maturation and Proliferation. In addition, these antibodies can damage megakaryocytes and/or at least block their release of platelets. Also, CD8+ cytotoxic T cells may damage platelets and megakaryocytes. All of these factors and viral infections, such as CMV, may contribute to reduced platelet production in ITP. The fact that thrombopoietin levels do not substantially increase in the setting of thrombocytopenia in ITP may also contribute to the failure to augment platelet production in many of these patients.
Studies Have Shown That Anti-Platelet Antibodies can Inhibit Megakaryocyte Maturation and Proliferation. In addition, these antibodies can damage megakaryocytes and/or at least block their release of platelets. Also, CD8+ cytotoxic T cells may damage platelets and megakaryocytes. All of these factors and viral infections, such as CMV, may contribute to reduced platelet production in ITP. The fact that thrombopoietin levels do not substantially increase in the setting of thrombocytopenia in ITP may also contribute to the failure to augment platelet production in many of these patients.
What about therapy? Has it been affected by these new advances? The most obvious change in the management of ITP has been the development and licensure of two thrombopoietic agents, romiplostim (AMG 531) and eltrombopag. There are now six peer-reviewed published studies on the use of these agents in ITP showing that they are unequivocally highly effective, tolerable, and not overly toxic.4-9 Even for refractory ITP, a response rate higher than 75 percent is anticipated. Responses are generally not seen until at least one to two weeks of therapy, depending upon dosage. Usually, it would take a minimum of four to six weeks of therapy at the highest dose to declare a patient “unresponsive.” ITP patients who have failed splenectomy are the best candidates for a TPO-R agonist, especially if they have also received rituximab. However, these agents are often used prior to splenectomy. The efficacy of these agents in chronic, refractory disease supports the pathophysiologic concept that ITP in part results from immune mediated suppression of platelet production. The potential toxicity of these agents is being monitored by the FDA-mandated Risk Evaluation and Mitigation Strategy (REMS) program, in which all cases are being tracked to determine the incidence of complications in larger numbers of patients than the 300 to 500 on whom licensure was based.
Other new therapeutic developments include use of more aggressive treatment upfront in newly diagnosed adults. This approach is based on the hypothesis that ITP is more likely to be “cured” by intensive immunosuppressive therapy early in its course (i.e., the newly diagnosed phase) than later on after the disease is well-established and patients have been exposed to multiple courses of treatment. The first attempt at this approach was the use of a single cycle of high-dose dexamethasone for four days.10 This was followed by further studies using three to four cycles11 of dexamethasone which suggested durable response rates of 60 to 80 percent. A recent study combined one cycle of dexamethasone with rituximab; the final results are not available yet. Studies comparing standard prednisone and dexamethasone are underway to clarify the utility of this approach.
This upfront treatment would be prior to consideration of splenectomy, the place of which is controversial at this time. It could be as early as three months from diagnosis after failure of the initial steroid treatment or only be resorted to after essentially all other active and safe agents have been tried. There are no clear data to inform this decision in that long-term data on splenectomy (e.g., for 10 to 30 years after) are largely non-existent.
ITP secondary to persistent infections (HIV, hepatitis C, H. pylori, and CMV) offers the opportunity to treat the infection as an approach to ameliorating or even curing the ITP. While HIV is usually clinically apparent, co-existing hepatitis C, H. pylori, and even CMV may not be in ITP patients. H. pylori is particularly confusing because it seems to be causative of ITP in Japan and Italy but much less so in the United States; this is based on the relative rates of improvement of ITP with eradication of the infection.12
Two important issues are fatigue and thrombophilia. Fatigue has now been shown in a number of studies to affect a substantial number of patients with ITP.13 Previously, this had been considered to be steroid-related, but it is clear now that it is an intrinsic part of ITP, more evident in certain patients than in others. As such, it may be appropriate to treat patients with minimal to no bleeding symptoms if they are substantially affected by fatigue (if treatment will improve fatigue). It has also become clear, although it remains not well studied, that ITP is simultaneously a pro-thrombotic disease as well as a hemorrhagic one. This means that the optimal platelet count is sometimes 50 to 100,000 μl instead of normal (i.e., >150,000 μl) and that patients with stable responses may require anti-platelet therapy.
Future developments will require more study of pathophysiology and especially how to apply emerging insights to patients. One striking example is the use of rituximab in ITP; in certain patients, 30 percent to 40 percent, there is a great response, while in others, no response whatsoever is seen.14 Controlled trials need to directly compare treatments to prospectively determine the pros and cons of common approaches, to investigate which is better, and to select treatments for individual patients.