The relationship between hemostasis and inflammation has emerged as a major area of investigation in recent years. The laboratory of Charles Esmon, in which the present work was performed, has played a major role in developing this area of study by unraveling mechanisms by which the serine protease, activated protein C (APC), functions. This effort led to the discovery by Esmon and Fletcher Taylor, a co-author of this paper, of APC as a potential therapeutic agent in the treatment of sepsis. Consequently, a recombinant APC product (drotrecogin alfa [activated]) was approved by the Food and Drug Administration in 2001 as the first treatment for adult patients with severe sepsis who are at high risk for death.
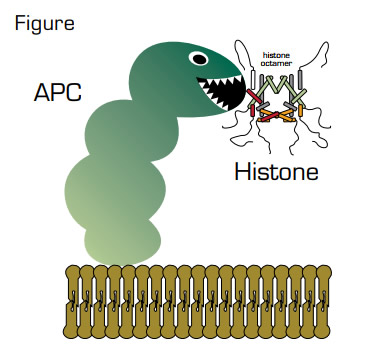
Several possible mechanisms have been identified to explain the anti-inflammatory functions of APC. The current study originated from the suspicion that the list of potential mediators of sepsis that are targeted by APC may be incomplete. To search for additional candidates, the authors exposed cultured endothelial cells to products released by a lipopolysaccharide- and interferon-γ-activated murine macrophage cell line. The releasate from these cells was cytotoxic toward the endothelial cells and addition of APC reduced the cytotoxicity. This finding instigated a hunt for putative substrates in the releasate that are targets for proteolytic degradation by APC and led to the identification of three small proteins with apparent molecular masses of 10, 13, and 15 kDa. Amino acid sequencing identified them as histones H4, H3, and H2A. Experiments using purified histone proteins confirmed that they are proteolytic substrates for APC and that cleavage decreases their cytotoxicity. Subsequent experiments revealed that that purified histones are cytotoxic toward endothelium in vitro and are lethal in mice. Anti-histone antibodies reduced the mortality in three models of murine sepsis. Additionally, histones were detected in the circulation of baboons subjected to Escherichia coli-induced sepsis. Co-infusion of APC with E. coli reduced mortality in baboons or mice. Finally, elevated histone levels were identified in the plasma of septic human patients, and cleaved H3 was observed in the plasma of one patient.
Histones are nuclear proteins that bind DNA and form nucleosomes, which are the primary components of chromatin. What are they doing outside of cells, and where do they come from? The findings in the present study are consistent with the identification of so-called neutrophil extracellular traps (NETs), which are partly made up of chromatin and are produced during sepsis, possibly to trap infectious agents.1
In Brief
This study provides compelling evidence that histones are primary mediators of sepsis and not merely a by-product. As noted by the authors, these results suggest new therapeutic approaches in the treatment of sepsis, such as the use of anti-histone antibodies or other histone blockers that could circumvent the bleeding complications that can result from APC therapy.
References
Competing Interests
Dr. Lollar indicated no relevant conflicts of interest.