In 1687, Isaac Newton postulated that during fluid flow parallel to a solid surface, fluid velocity increases proportionately with distance from the surface. The idea is that a layer of fluid at the surface sticks to the surface, a layer of fluid on top of that feels the opposing forces of being stuck to the layer below it and being pulled along by the layer moving above it, and so on. This spatial dependence of fluid velocity is called the shear rate, and the force that produces it is called shear stress. How could a platelet 2 microns in diameter traveling in the center of an arteriole 30 microns in diameter ever find its way to a site of vascular injury at the vessel wall? It has been known for nearly 40 years that shear stress plays an important role in the interaction of platelets with surfaces.1-4 For a platelet to stick to the vessel wall (adhesion) or to the surface of another platelet (aggregation), it must be transported to the site by a combination of flow and radial diffusion (i.e., diffusion perpendicular to the flow). In whole blood, the latter increases as shear rate increases.5 After reaching the surface, platelets bind via inter-molecular interactions; e.g., platelet GPIb binds to von Willebrand factor. If binding is rapid relative to transport, then the rate of adhesion and aggregation is dominated by factors that influence transport. The extent of platelet aggregation under flow conditions undergoes a shear-dependent optimum that is transport-limited at low shear rates and reaction-limited at higher shear rates.4
Standing on the shoulders of giants so to speak, Nesbitt et al. in the laboratory of Shaun Jackson used a combination of in vivo intravital imaging of the murine mesenteric arteriolar circulation, invitro microfluidic flow studies, and computational fluid dynamics to obtain additional insights into the nature of platelet aggregation and thrombus formation. In contrast to the earlier studies cited above, they progressively stenosed arterioles at the site of a crush injury or fabricated microchannels with similar stenotic geometry and measured the kinetics and size of thrombus formation. As in previous studies, they found that platelet aggregation in a growing thrombus is primarily driven by shear. Additionally, they made the novel observation that thrombus formation is a function of local changes in shear, termed shear microgradients, that are produced by vessel geometry. In contrast, they found that platelet activation produced by soluble agonists plays a secondary role by stabilizing aggregates.
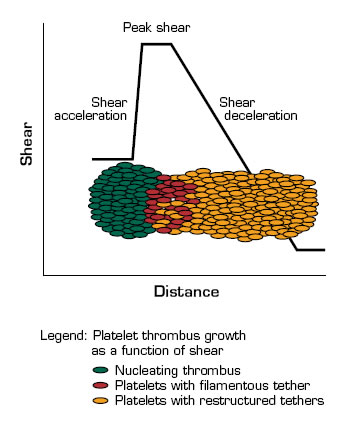
The shear microgradients that they produced consist of acceleration, peak, and deceleration zones. At peak shear, platelets adhere to exposed thrombogenic surfaces in a GPIb-dependent manner. The shear on these platelets results in the extrusion of thin filamentous membrane tethers, which facilitates the recruitment of platelets into the downstream deceleration zone. There, the reduced shear favors the formation of αIIbβ3-dependent aggregation that results in tether restructuring and the stabilization of the aggregates. In this process, ongoing platelet recruitment drives the propagation of the thrombus in the downstream deceleration zone, which may, in turn, amplify the shear microgradient and promote further platelet aggregation. The shear-dependent formation of membrane tethers brings together the physics (transport and diffusion under flow) and chemistry (membrane stickiness) of platelet function.
In Brief
This study provides new insights into the potentially prothrombotic effects of blood flow at sites of stenosis. Continued investigation of the dependence of platelet kinematics, membrane restructuring, and aggregation on blood flow and vessel geometry may lead to the development of safer and more effective antithrombotic therapies.
References
Competing Interests
Dr. Lollar indicated no relevant conflicts of interest.