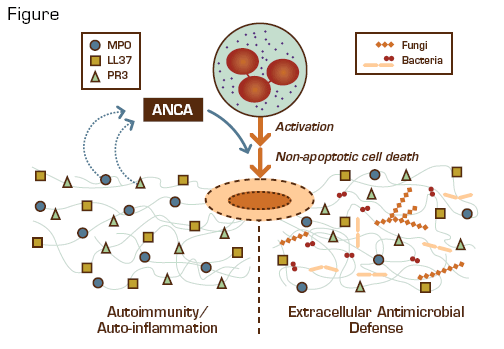
The critical roles of neutrophils in innate immune responses are mediated by oxidative and non-oxidative mechanisms. In 2004, another weapon in the neutrophil arsenal was identified: neutrophil extracellular traps (NETs).1 NETs are extracellular web-like structures of chromatin, DNA, and granular proteins that are triggered by reactive oxygen species produced by NADPH oxidase in activated neutrophils and are deployed during non-apoptotic cell death. NETs entrap and kill bacteria and fungi. They have also been implicated in "friendly fire damage," including preeclampsia (by obstructing placental blood flow), infertility (by ensnaring sperm in the reproductive tract), cystic fibrosis (by contributing DNA to alveolar plugs), and sepsis. Because NETs contain nuclear and chromatin-associated proteins, their extracellular deposition has been postulated to induce anti-nuclear antibodies (ANA) and anti-neutrophil cytoplasmic antibodies (ANCA) against myeloperoxidase (MPO; p-ANCA) and proteinase-3 (PR-3; c-ANCA).
Neutrophil Extracellular Traps (NETs) Provide an Important Antimicrobial Defense Mechanism but Might Also Play a Deleterious Role in the Pathogenesis of ANCA-Mediated Small Vessel Vasculitis and Tissue Injury.
Neutrophil Extracellular Traps (NETs) Provide an Important Antimicrobial Defense Mechanism but Might Also Play a Deleterious Role in the Pathogenesis of ANCA-Mediated Small Vessel Vasculitis and Tissue Injury.
Kessenbrock et al., working with Dieter Jenne and Volker Brinkmann at the Max Planck Institute in Germany, investigated the potential role of NETs in the pathogenesis of ANCApositive, small-vessel vasculitis (SVV). They observed that purified IgG obtained from individuals with SVV and murine anti-PR3 monoclonal antibodies both efficiently induced NET formation by neutrophils primed with tumor necrosis factor-α. Sera from patients with SVV reacted strongly with NETs induced by the phorbal ester PMA, and immunofluorescence confirmed colocalization of NETs with MPO, PR-3, and LL37 (an anti-microlocalization of DNA, histones, MPO, PR-3, and LL37 in glomeruli infiltrated with neutrophils. Moreover, a marker associated with local interferon-α production was increased in the periglomerular tissue, consistent with an auto-inflammatory process. Finally, circulating MPO-DNA complexes were found in sera from patients with active SVV.
In Brief
As with many pro-inflammatory processes, adverse end-organ consequences can occur if the innate immune response is overactive, prolonged, or lacks appropriate regulatory controls. This study suggests that NETs can initiate and contribute to autoimmune SVV by exposing self-antigens that induce auto-antibodies, which, in turn, trigger activated neutrophils to deploy additional NETs, promote inflammation, and injure tissue. It remains unclear how NET production might generate such autoimmune responses. Bacterial infection could play a role if cross-reactivity exists between NET and microbial antigens, similar to mechanisms described for pauci-immune necrotizing glomerulonephritis.2 Also, since neonatal neutrophils lack the ability to produce NETs,3 tolerance or autoimmunityto NET antigens may be determined during evolution of adaptive immunity in early childhood. It is intriguing to speculate that individuals unable to form NETs, such as those with chronic granulomatous disease, might be at increased risk for generating ANA or ANCA and developing autoimmune disease after restoration of NET function by gene therapy.4 NET formation clearly plays a beneficial role in extracellular antimicrobial defense. However, the potential for collateral damage mandates further investigation to better understand how the NET weapon develops and how it might "misfire" in order to implement and evaluate strategies to regulate its function.
References
Competing Interests
Drs. Hobbs and Linenberger indicated no relevant conflicts of interest.