Most of the trypsin-like serine proteases in the coagulation and fibrinolytic mechanisms are formed by proteolytic activation from an inactive precursor, called the zymogen. During this process, a peptide at the NH2-terminus of the zymogen is cleaved. In a process referred to as molecular sexuality, the newly formed NH2-terminus of the nascent enzyme forms an internal salt bridge that results in conformational activation of the catalytic site. Prothrombin (proT) normally is proteolytically activated to thrombin by factor Xa. However, staphylocoagulase, a secretory product of Staphylococcusaureus, activates prothrombin non-proteolytically. In 2003, Friedrich et al., from the laboratory of Paul Bock at Vanderbilt University, reported that staphylocoagulase accomplishes this by inserting a dipeptide from its NH2-terminus into proT to substitute for the normal internal salt bridge.¹
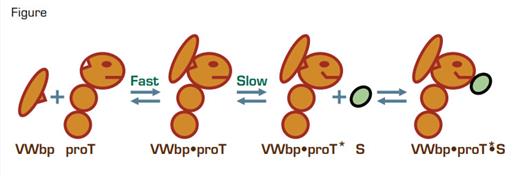
Von Willebrand factor-binding protein (vWbp) is another secretory product of S. aureus and is homologous to staphylocoagulase. Kroh et al., from the Bock laboratory, nowhave found that vWbp also is a potent activator of proT. Like staphylocoagulase, vWbpnon-proteolytically activates proT by a molecular sexuality mechanism. However, unlikestaphylocoagulase, there is a distinct lag period between the addition of vWbp to proTand the development of enzymatic activity. Approximately 40 years ago, Carl Frieden usedthe term hysteresis, from the Greek meaning “lagging behind,” to describe a mechanismin which enzymes develop activity only after a slow conformational change that follows theformation of the initial enzyme-substrate complex.² Bock trained under Frieden, and, thus,he and his co-workers were prepared to determine whether the vWbp-proT complex is ahysteretic enzyme. They found that the behavior of the vWbp-proT complex indeed fit akinetic model consistent with the hysteretic mechanism. In the model, vWbp binds rapidlyto proT. The resulting complex slowly equilibrates into an active vWbp-proT* complex that binds the substrate, S, with high affinity (Figure). There is a lag phase as the substrate (e.g., fibrinogen) waits for the active vWbp-proT* complex to form. Once formed, the fibrinogen rapidly latches on and is converted to product.
Kroh et al. propose that during staphylococcal endocarditis vWbp binds to von Willebrand factor that has been deposited at sites of vascular injury. Because a substrate is required in vivo to pull the vWbp-proT complex toward the active state, they suggest that the hysteretic mechanism restricts activation of proT by vWbp to areas rich in the substrate, fibrinogen. Thus, the initial formation of fibrin on infected heart valves may be catalyzed by vWbp-proT, followed by additional staphylocoagulase-mediated fibrin deposition and vegetation growth.
In Brief
This study is important because it describes a novel enzyme, vWbp-proT, and rigorously tests a kinetic mechanism that accounts for the lag behavior displayed by the enzyme. This behavior is then interpreted in the context of an important disease setting, staphylococcal endocarditis.
References
Competing Interests
Dr. Lollar indicated no relevant conflicts of interest.