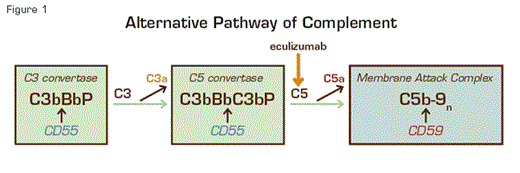
The chronic intravascular hemolysis that is the hallmark clinical manifestation of paroxysmal nocturnal hemoglobinuria (PNH) is mediated by the alternative pathway of complement (APC) (Figure 1). Because the antibody-independent APC is primed for attack at all times, elaborate mechanisms for self-recognition and protection of the host against complement-mediated injury have evolved. Both fluid-phase and membrane-bound proteins are involved in these protective and self-recognition processes. Normal human erythrocytes are protected against APC-mediated destruction primarily by decay accelerating factor (DAF, CD55) and membrane inhibitor of reactive lysis (MIRL, CD59). These proteins act at different steps in the complement cascade (Figure 1). CD55 regulates the formation and stability of the C3 and C5 convertases (Figure 1, inside green boxes), while CD59 blocks the formation of the membrane attack complex (MAC) (Figure 1, inside blue box). Deficiency of both CD55 (brown ovals) and CD59 (gold ovals) on the affected erythrocytes is the pathophysiologic basis of the Coombs’-negative, intravascular hemolysis that defines PNH (Figure 2).
Complement-Mediated Lysis of PNH Erythrocytes. The C3 convertase (left, green box) of the APC consists of a multiprotein complex of activated C3 and B (C3b and Bb), and the stabilizing cofactor P. The C5 convertase (right, green box) has the same components as the C3 convertase except that it includes 2 copies of C3b. The C3 and C5 convertases generate the active peptides C3a and C5a along with the MAC, which consists of C5b, C6, C7, C8, and multiple molecules of C9, and which functions as a cytolytic unit (blue box). The glycosyl-phosphatidylinositol (GPI)-anchored complement regulatory protein CD55/DAF restricts formation and stability of both the C3 and the C5 convertases (brown arrows), whereas GPI-anchored CD59/MIRL blocks formation of the MAC (brown arrow). Inhibition of MAC formation by anti-C5 monoclonal antibody eculizumab (gold arrow) ameliorates the intravascular hemolysis of PNH.
Complement-Mediated Lysis of PNH Erythrocytes. The C3 convertase (left, green box) of the APC consists of a multiprotein complex of activated C3 and B (C3b and Bb), and the stabilizing cofactor P. The C5 convertase (right, green box) has the same components as the C3 convertase except that it includes 2 copies of C3b. The C3 and C5 convertases generate the active peptides C3a and C5a along with the MAC, which consists of C5b, C6, C7, C8, and multiple molecules of C9, and which functions as a cytolytic unit (blue box). The glycosyl-phosphatidylinositol (GPI)-anchored complement regulatory protein CD55/DAF restricts formation and stability of both the C3 and the C5 convertases (brown arrows), whereas GPI-anchored CD59/MIRL blocks formation of the MAC (brown arrow). Inhibition of MAC formation by anti-C5 monoclonal antibody eculizumab (gold arrow) ameliorates the intravascular hemolysis of PNH.
The complement-mediated intravascular hemolysis of PNH can be inhibited by blocking formation of the MAC. The MAC consists of complement components C5b, C6, C7, C8, and multiple molecules of C9. Eculizumab is a humanized monoclonal antibody that binds to complement C5, preventing its activation to C5b and thereby inhibiting MAC formation (Figure 1). In 2007, eculizumab was approved by both the FDA and the European Union Commission for treatment of the hemolysis of PNH. Treatment with eculizumab reduces transfusion requirements, ameliorates the anemia of PNH, and improves quality of life by attenuating the debilitating constitutional symptoms (fatigue, lethargy, asthenia) associated with chronic complement-mediated intravascular hemolysis. Following treatment, serum lactate dehydrogenase concentration (LDH), a surrogate marker for intravascular hemolysis (Figure 2), returns to normal, but mild to moderate anemia, hyperbilirubinemia and reticulocytosis often persist.1 This could be the result of ongoing extravascular hemolysis of PNH erythrocytes as a consequence of C3 opsonization as eculizumab does not block the activity of the APC C3 convertase (Figure 1).
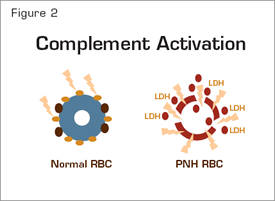
Support for this hypothesis is provided by the recent studies of Antonio Risitano and colleagues from Italy. By using two-color flow cytometric analysis, the investigators showed that, in patients treated with eculizumab, a portion of the PNH erythrocytes (i.e., the CD59-deficient population) had C3 bound. The studies of Risitano et al. also confirmed the Coombs’-negative designation of PNH, as no C3 was found bound to PNH erythrocytes prior to initiation of treatment with eculizumab, consistent with a model in which, in the absence of eculizumab, PNH erythrocytes upon which complement has been activated are destroyed directly as a consequence of MAC-mediated cytolysis (Figure 2). Post hoc analysis of their data suggested that the percentage of C3+ erythrocytes negatively influenced response (based on steady-state hemoglobin concentration, transfusion requirement, and LDH concentration) in patients treated with eculizumab, but considerable overlap in the percentage of C3+ erythrocytes among the designated treatment groups was observed.
Complement Activation. Normal erythrocytes (left) are protected against complement-mediated lysis primarily by CD55 (brown circles) and CD59 (gold circles). Deficiency of these GPI-anchored complement regulatory proteins results in APC activation on PNH erythrocytes (right). Consequently, MACs form pores in the red cell membrane resulting in osmotic lysis and release of hemoglobin and LDH into the intravascular space.
Complement Activation. Normal erythrocytes (left) are protected against complement-mediated lysis primarily by CD55 (brown circles) and CD59 (gold circles). Deficiency of these GPI-anchored complement regulatory proteins results in APC activation on PNH erythrocytes (right). Consequently, MACs form pores in the red cell membrane resulting in osmotic lysis and release of hemoglobin and LDH into the intravascular space.
So, is there a better way to treat PNH? Why not block C3 and thereby inhibit not only MAC-mediated lysis, but also C3 opsonization? The major problems with this therapeutic approach are the high concentration of C3 in plasma and, more importantly, the central role that C3 plays in both the classical and the lectin pathways of complement as well as the APC. Blocking all three complement pathways would almost certainly result in unacceptable toxicity as congenital deficiency of C3 is associated with recurrent bacterial infection and early mortality. Still, it may be possible to target only the APC by blocking factor B (Figure 1) or by developing antibodies that inhibit APC C3 convertase formation without affecting C3 function within the classical and lectin pathways.
In Brief
Identification and characterization of the supernormal binding of C3 to PNH erythrocytes in 1973 by Logue, Rosse, and Adams2 was a watershed event that led to the discovery of DAF- deficiency and, ultimately, to an understanding of the molecular basis of PNH. Treatment with eculizumab alters the natural history of the disease by converting the hemolysis of PNH from a Coombs’-negative process to a Coombs’-positive process (at least in some patients). Interestingly, this “new” observation was predicted by discoveries made long before current treatment of PNH was imagined.
References
Competing Interests
Dr. Parker indicated no relevant conflicts of interest.