Numerous studies suggest that platelets function in antimicrobial host defense. They accumulate at sites of endothelium damaged by bacterial or fungal colonization. They bind and engulf viruses (e.g., HIV-1, HCV). They store and secrete antimicrobial proteins. They interact with leukocytes, augmenting their activity during microbial infections. But really, how important are platelets in fighting infection?
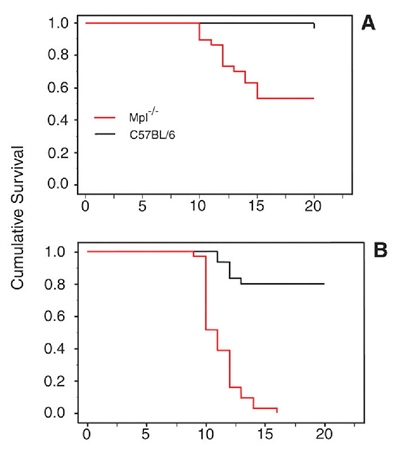
New evidence indicates that, in the response to plasmodial invasion, platelets may be very important. While evaluating mice for genetic mutations that render them resistant to malaria, McMorran, et al., from the Menzies Research Institute at University of Tasmania, serendipitously discovered that thrombocytopenic mice were unusually susceptible to infection by Plasmodium chabaudi. They subsequently tested survival following P. chabaudi infection in wild-type and Mpl-/- mice, which lack the thrombopoietin receptor and have ~15 percent as many platelets as their wild-type counterparts.1 Nearly all (95%) of wild-type female mice survived the infection, compared to only 50 percent of Mpl-/- mice (see Figure). In males, 80 percent of wild-type mice survived, while all the Mpl-/- mice died. These results suggest that platelets are protective against malarial infection.
Survival Following P. Chabaudi Infection in Wild-Type and Mpl-/- Mice. From McMorran BJ, Marshall VM, de Graaf C, et al. Platelets kill intraerythrocytic malarial parasites and mediate survival to infection. Science. 2009;323: 797-800. Reprinted with permission from AAAS.
Survival Following P. Chabaudi Infection in Wild-Type and Mpl-/- Mice. From McMorran BJ, Marshall VM, de Graaf C, et al. Platelets kill intraerythrocytic malarial parasites and mediate survival to infection. Science. 2009;323: 797-800. Reprinted with permission from AAAS.
Evaluation of the peripheral blood smears from wild-type mice following P. chabaudi infection demonstrated that three times as many infected erythrocytes were bound by platelets compared to uninfected erythrocytes. Parasitemia rates were twice as high in platelet-bound erythrocytes compared with all erythrocytes, raising the possibility that platelets recognize infected erythrocytes. Early in the infection (day 7), twice as many dead intraerythrocytic parasites were seen in wild-type mice as in Mpl-/- mice. This observation correlated with the finding that, at days 8 and 9, significantly higher levels of total parasitemia were observed in Mpl-/- mice compared with wild-type. Thus, platelets appear to act early in the course of P. chabaudi infection in order to limit the extent of parasitemia.
Studies performed in vitro using human platelets demonstrated that platelets inhibited P. falciparum growth in a dose-dependent manner. More than three times as many dead parasites were observed in cultures incubated with platelets, suggesting that platelets actively kill the intraerythrocytic parasites. The inhibitory activity of platelets was blocked by several antiplatelet compounds, including aspirin. Mice treated with aspirin were less likely to survive P. chabaudi infection than untreated mice. Thus, antiplatelet agents interfere with the protective effect of platelets.
In Brief
Approximately half a billion cases of malaria occur annually, killing 1 to 3 million people each year. Platelets had previously been shown to complicate malarial infection by sequestering erythrocytes in the cerebral vasculature. McMorran, et al. now find an independent and beneficial function of platelets in limiting the extent of parasitemia following plasmodial infection. The findings of McMorran, et al. that platelets may serve a protective function in malaria have implications with regard to how patients are presently managed as well as for future strategies in treating this disease. For example, aspirin is commonly used as an antipyretic in the context of malarial infections. Might this practice be detrimental to the patient? Could maintaining normal platelet counts be useful in controlling malarial infections?
Several basic questions arise from this work as well. What are the signals that enable platelets to recognize infected erythrocytes? How do bound platelets kill intraerythrocytic parasites? As elegant and novel as this study is, the results of McMorran, et al. offer little information with regard to mechanism. Elucidation of the mechanisms by which platelets kill plasmodium may lead to the development of new strategies and reagents for fighting malaria.
References
Competing Interests
Dr. Flaumenhaft indicated no relevant conflicts of interest.