At the 2007 ASH annual meeting in Atlanta, Ernest Beutler was scheduled to present another amazing hematologic breakthrough during the plenary session. Because of his ill health, his son Bruce presented pictures of a semi-hairless mouse with anemia, microcytosis, iron deficiency, and high hepatic levels of hepcidin mRNA transcripts.1 In response to high doses of iron, they grew hair. (Some iron-deficient humans have hair loss.) This so-called “mask” mouse could not absorb iron from the gut (most likely due to the high hepcidin levels) and had a mutation on chromosome 15 in a gene encoding a transmembrane serine protease of unknown function, TMPRSS6 or matriptase-2. Dr. Beutler’s team demonstrated that the protein encoded by the normal TMPRSS6 gene suppressed hepatic hepcidin expression induced by bone morphogenic protein (BMP), hemojuvelin (HJV), SMAD-1, and IL-6, while the protein encoded by the mutated mask allele or a mutated protein with an inactive protease domain did not suppress. They concluded that TMPRSS6 is required to sense iron deficiency, but its sensing mechanism remained speculative.2
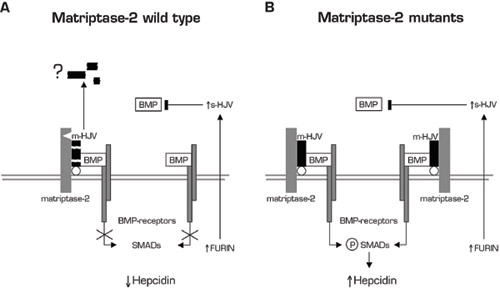
1. Schematic representation of a model of matriptase-2 activity in iron deficiency. On the left, the serine protease cleaves m-HJV releasing soluble fragments (here simplified by the black boxes). The cleavage sites of matriptase-2 are unknown. The question mark indicates uncertainty on fragments’ function. The resulting hepcidin inhibition is shown. The complementary effect of s-HJV, produced by furin cleavage, to sequester BMP is shown on the right. 2. Lack of hepcidin inhibition in the presence of matriptase-2 mutations. m-HJV acts as BMP co-receptor and permits hepcidin production in iron deficiency; the effect of s-HJV cannot down-regulate hepcidin in the presence of m-HJV. Reprinted from Silvestri L, Pagani A, Nai A, et al. The serine protease matriptase-2 (TMPRSS6) inhibits hepcidin activation by cleaving membrane hemojuvelin. Cell Metabolism. 2008;8:502-11. With permission from Elsevier.
1. Schematic representation of a model of matriptase-2 activity in iron deficiency. On the left, the serine protease cleaves m-HJV releasing soluble fragments (here simplified by the black boxes). The cleavage sites of matriptase-2 are unknown. The question mark indicates uncertainty on fragments’ function. The resulting hepcidin inhibition is shown. The complementary effect of s-HJV, produced by furin cleavage, to sequester BMP is shown on the right. 2. Lack of hepcidin inhibition in the presence of matriptase-2 mutations. m-HJV acts as BMP co-receptor and permits hepcidin production in iron deficiency; the effect of s-HJV cannot down-regulate hepcidin in the presence of m-HJV. Reprinted from Silvestri L, Pagani A, Nai A, et al. The serine protease matriptase-2 (TMPRSS6) inhibits hepcidin activation by cleaving membrane hemojuvelin. Cell Metabolism. 2008;8:502-11. With permission from Elsevier.
Now, Silvestri, et al., from Clara Camaschella’s group in Milan, report that matriptase-2 inhibits hepcidin activation by cleaving membrane HJV, thus interfering with primary hepcidin signaling in the hepatocyte. To review, hepcidin levels are regulated by body iron levels. Inflammation and cytokines such as IL-6, erythroid factors such as growth differentiation factor 15 (GDF15), and hypoxia via hypoxia-inducible transcription factors all play roles in modulating hepcidin synthesis. Hepatic hepcidin regulation by iron is regulated by HJV, which acts as a co-receptor for BMP to the BMP receptor, signaling a cascade to SMAD-4 translocation to the nucleus and hepcidin transcription. HFE, TfR2, and transferring are critical to this process.3
In an elegant set of studies, Silvestri, et al. demonstrate that matriptase-2 cleaves membrane HJV releasing proteolytic fragments and disrupts the HJV/BMP complex binding to the BMP receptor, inhibiting hepcidin signaling (see Figure). The mask mutant matriptase-2 and a partially mutated matriptase-2 from a family with refractory iron deficiency did not cleave HJV and, when expressed in zebrafish, caused anemia. Soluble HJV cleaved in vitro by furin (distinct from membrane HJV) can act as a decoy receptor competing for membrane HJV, thus decreasing hepcidin. Matriptase-2 does not cleave soluble HJV.
These studies are relevant in both iron deficiency and iron overload. Two recent publications reported mutations in the TMPRSS6 in humans associated with familial iron deficiency refractory to oral iron.4,5 Thus, these translational studies extend our understanding of iron metabolism. Unmasking the role of this protease, matriptase-2, adds another brick in the foundation of hematology that our late ASH president, Ernest Beutler, had so importantly helped build.
References
Competing Interests
Dr. Vercellotti indicated no relevant conflicts of interest.