The studies in this manuscript significantly contribute to the solution of two scientific puzzles. They elucidate the mechanism of the (surprisingly) essential role of growth factor independence 1 (Gfi1), which was originally identified as an oncogene, in neutrophil differentiation; and by dissecting the mechanism by which a mutant form of GFI1 leads to severe congenital neutropenia (SCN), they generate the long sought-after mouse model for this human disease, hopefully leading to further understanding and treatment.
Gfi1 derives its name from its identification as an oncogene by an insertional mutagenesis screen. Gfi1 was found to be over-expressed in T-cell lymphomas, suggesting that Gfi1 should be down-regulated during normal hematopoietic differentiation. However, as with many other genes, it’s not that simple. Gfi1 functions as an oncogene in some cell types, while promoting differentiation in others. The discovery that Gfi1 knockout mice have many developmental defects, including severe neutropenia,1 prompted the identification of mutations in this gene in patients with SCN. Gfi1 is a transcriptional repressor, which binds to its specific target DNA sequences via its 5 zinc finger domains. The SCN-associated GFI1N382S mutation (the asparagine at position 382 is replaced by a serine) occurs in the 5th zinc finger and completely prevents DNA binding.
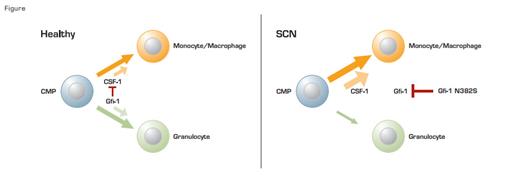
In healthy individuals, Gfi1 regulates the balance between granolocytic and monocytic differentiation by maintaining low levels of CSF-1. In patients with SCN, the mutant form of Gfi-1 (N382S) inhibits normal Gfi-1 function leading to decreased granulocytic and increased monocytic differentiation. The monocytes formed, however, are abnormal.
In healthy individuals, Gfi1 regulates the balance between granolocytic and monocytic differentiation by maintaining low levels of CSF-1. In patients with SCN, the mutant form of Gfi-1 (N382S) inhibits normal Gfi-1 function leading to decreased granulocytic and increased monocytic differentiation. The monocytes formed, however, are abnormal.
Zarebski, et al. validate and further elucidate the mechanism by which wildtype Gfi1 promotes, and mutated GFI1N382S inhibits, granulocytic differentiation. Previously published data show that Gfi1 is required for normal terminal differentiation of granulocytes.1 Myeloid cells lacking GFI1 are blocked at an abnormal stage of differentiation wherein they have features of both neutrophils (Gr1 expression and the ability to mount a respiratory burst) and macrophages (Mac1 expression and phagocytosis), but lack secondary granules and do not adequately respond to bacterial infection. In their manuscript, Zarebski, et al. show that, as expected, enforced expression of exogenous Gfi1 in hematopoietic progenitor cells leads to an increase in neutrophils and a decrease in monocytes, while Gfi1-null progenitors differentiate predominantly into monocytes and a "neutrophil-monocyte mix cell" but no neutrophils. Expression of the mutant GFI1 (N382S) fails to correct the null phenotype and mimics the null phenotype when overexpressed in wild-type progenitors, demonstrating that it is a dominant negative mutation. Zarebski, et al. identified several potential target genes of Gfi1 that may mediate this effect, but only colony stimulating factor-1 (CSF-1) fulfilled two criteria making it a likely candidate gene in SCN: Its expression is down-regulated by GFI1, and it is de-repressed (i.e., ultimately up-regulated) by GFI1N382S. To demonstrate the importance of CSF-1 as a target gene, Zarebski, et al. showed that hematopoietic progenitor cells from CSF-1 null mice undergo normal granulocytic differentiation even when induced to express GFI1N382S.
In Brief
These results clarify that inhibition of GFI1’s inhibition (inhibition + inhibition = de-repression) of monocyte differentiation by a mutant dominant negative GFI1 plays a role in the pathogenesis of SCN and suggest that inhibition of CSF-1 activity in vivo could provide therapeutic benefit. How GFI1 promotes terminal differentiation of granulocytes beyond the "biphenotypic" fate decision of granulocyte/monocyte precursors is not yet clear. Perhaps identification of additional GFI1N382S targets in the future will lead to the development of therapies that fully promote functional granulocytes in patients with SCN.
References
Competing Interests
Drs. Halene and Krause indicated no relevant conflicts of interest.