Leukemia-initiating cells (LICs; also known as leukemia stem cells) have been identified in myeloid and lymphoid acute and chronic leukemia subtypes. Like normal hematopoietic stem cells (HSCs), LICs are pluripotent, self-renewing, phenotypically primitive and mitotically quiescent. Their non-cycling status and inherent or acquired drug resistance mechanisms allow them (like HSCs) to escape conventional and targeted therapies that effectively kill the more mature, proliferating leukemia cell progeny. LICs, therefore, serve as a reservoir for disease persistence and relapse. One theoretic approach to eliminate LICs and improve cure rates is to induce their entry into cell cycle to promote differentiation and clonal extinction and/or to render them susceptible to cytotoxic agents.
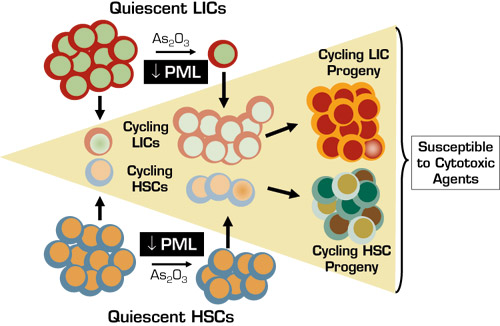
The study by Ito, et al. was prompted by observations of high levels of promyelocytic leukemia (PML) tumor suppressor protein in blast cells in 88 percent of chronic-phase CML cases and an association between high-blast PML expression and poor response to imatinib. They sought to understand the role of PML in normal HSC maintenance and whether it might serve as a useful therapeutic target in LICs. High PML mRNA and protein levels were found in normal human and murine HSCs but not in more committed progenitors. PML absence (in Pml–/– mice) or pharmacologic down-modulation (using arsenite arsenic trioxide [As2O3]) was associated with increased cell cycling and ultimate "exhaustion" of HSCs as manifested by compromised long-term engraftment post-transplant and impaired reconstitution with serial transplantation. CML LICs were generated by transduction of p210BCR-ABL into HSCs from Pml+/+ and Pml–/– mice. Culture studies and in vivo models revealed that PML was required for LIC mitotic quiescence, long-term maintenance, and aggressive disease phenotype in serially transplanted mice. Additional studies indicated that PML acts as a repressor of mTOR and that mTOR super-activation can impair HSC and LIC maintenance, but inhibition of mTOR with rapamycin accentuates in vivo lethality of Pml+/+ CML LICs. Like HSCs, As2O3 induced quiescent Pml+/+ LICs into cell cycle and led to clonal extinction during serial transplantation.
As2O3 also profoundly augmented cytosine arabinoside (Ara-C)-induced apoptosis, but the effects on normal murine HSCs were modest compared to LICs. Thus, mice with experimental CML could eventually be "cured." In vitro studies with human cells showed similar pronounced and preferential effects of As2O3 alone and in combination with Ara-C on PML expression, cell cycle entry, maintenance, and drug susceptibility in primary CML LICs compared to normal HSCs.
In Brief
This study provides a number of key insights: 1) PML is an essential regulator of HSC mitotic quiescence and long-term maintenance; 2) PML is required for CML LIC quiescence and disease persistence in murine models; and 3) PML down-regulation in CML LICs by As2O3, a commercially available drug, preferentially and potently induces entry into cell cycle and drug sensitization (see Figure), thereby providing an immediately testable therapeutic approach.
Enthusiasm must be tempered by the fact that genomic instability in CML LICs leads to additional molecular lesions besides BCR-ABL1, and, therefore, pharmacologic PML manipulation may not yield the predicted effects in clinical trials. To fully exploit PML as a therapeutic target, it will also be necessary to define the relevant mechanistic pathways and proteins (in addition to mTOR) involved in HSC maintenance and LIC pathophysiology. This will be challenging because PML is a critical component of the PML nuclear body that regulates DNA damage responses, apoptosis, cellular senescence, and angiogenesis through a variety of complex multiprotein interactions that are not yet well characterized. Lastly, it will be important to determine whether PML plays a similar role in LICs in other leukemias, or in non-hematologic tissue stem cells and cancer-initiating cells, and to explore potential therapeutic applications beyond CML.
Competing Interests
Dr. Linenberger indicated no relevant conflicts of interest.