Drs. Halene and Krause indicated no relevant conflicts of interest.
Hanna J, Wernig M, Markoulaki S, et al. Treatment of sickle cell anemia mouse model with iPS cells generated from autologous skin. Science. 2007;318:1920-3.
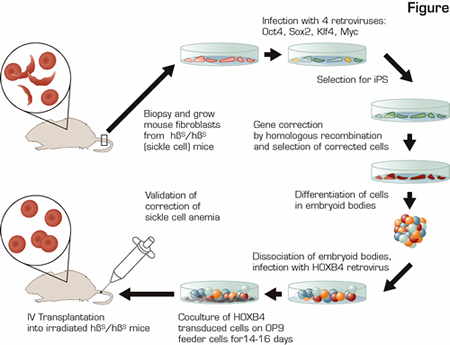
In the research reported, the investigators combined multiple state-of-the-art advances in stem cell and gene therapy research to cure mice with sickle cell anemia (SCA) using gene-corrected syngeneic (analogous to autologous) somatic cells derived from skin fibroblasts. This was a heroic feat as shown in the schematic diagram (see Figure). They generated inducible pluripotent stem (iPS) cells from skin of the sick animals, repaired the point mutation causing SCA, differentiated the gene-corrected iPS cells down the hematopoietic lineage, and transplanted the iPS-derived, corrected hematopoietic cells into the SCA mice to provide long-term engraftment of the hematopoietic system yielding plenty of RBCs expressing normal ß globin.
The most recent advance in stem cell biology allowing this success was the development of iPS cells. About one year ago,1 the laboratory of Shinya Yamanaka published findings that a combination of four transcription factors inserted permanently into the genomic DNA of mouse fibroblasts using retroviral infection could reprogram the fibroblasts to become embryonic stem (ES) cell-like cells, termed iPS cells. As of January 2008, there were 12 research papers published on iPS cells, all confirming that iPS cells are phenotypically and functionally very similar to embryonic stem cells.
In this most recent manuscript, the investigators used a mouse model of human SCA in which the endogenous mouse globin genes have been replaced with human α and ß globin genes, but instead of inserting human ßA, which encodes normal ß globin, these mice have the human ßS gene, which causes homozygous ßS/ßS mice to have SCA. Fibroblasts from a simple tail biopsy of a ßS/ßS mouse were grown in vitro and transformed into iPS cells by infection with four retroviruses encoding the transcription factors Oct4, Sox2, Klf4, and c-Myc. Once the iPS culture was established, the Myc transgene was removed by targeted recombination to decrease the risk of malignant transformation without loss of the ES-like qualities. Subsequently, the ßS mutation in these iPS cells was corrected by homologous recombination with a DNA template containing the normal ßA sequence along with two additional genes to allow for selection of this rare recombination event. Importantly, iPS technology has opened an avenue where hematopoietic stem cells (HSCs) have failed. In vitro selection for corrected HSCs cannot be performed, because, as opposed to iPS cells, HSCs lose their "stem-cell-ness" during the long-term culture (weeks) necessary for selection of corrected cells. The "corrected" iPS cells were then differentiated into early blood cells via: a) formation of embryoid bodies that contain multiple cell types including blood cells; b) infection with a retrovirus carrying the transcription factor HOXB4 (necessary for ES- or iPS-derived cells to engraft as HSCs in vivo);2 and c) expansion for 14 to 17 days on an irradiated feeder cell layer. Only then were the cells transplanted back into irradiated SCA mice. In three of three transplanted ßS/ßS mice, iPS-derived cells contributed to 40 percent to 70 percent of the peripheral blood cells, ßA globin was detected at the RNA and protein levels, and the RBC counts and hemoglobin in the peripheral blood were significantly increased compared to untransplanted ßS/ßS mice for at least three months. It remains to be determined how long this engraftment will last, but mice with donor-cell engraftment at three months generally have stable long-term engraftment.
In Brief
In summary, the investigators took a multi-step approach to perform cell and gene therapy for SCA. While this required infection with five different retroviruses as well as electroporation of the cells with the DNA template for normal human ß globin, this is a proof-of-principle paper that represents the potential future of autologous cell and gene therapy. Theoretically, once investigators develop approaches to reprogram these cells without the need for permanent retroviral insertion, this could be applied for treatment of patients with multiple genetic disorders of the hematopoietic system.