Just over a 100 years ago, James Homer Wright, observing that platelets shared tinctorial properties with giant bone marrow cells (now termed megakaryocytes), postulated that platelets were fragments of these large cells. Two models have since evolved to explain how platelets are derived from megakaryocytes. The flow model proposes that megakaryocytes elaborate long pseudopodia that bend, branch, and bulge. The bulges that develop along the length and ends of these pseudopodia form proplatelets, which mature into platelets. The platelet territory model proposes that megakaryocytes fragment along internal demarcation membranes to form platelets. These models were based largely on either analysis of high-resolution snapshots of thrombopoiesis or by observations of cultured megakaryocytes. But how does thrombopoiesis actually occur in the bone marrow?
In a recent article in Science, Junt and colleagues address this question by viewing thrombopoiesis directly in living mice. The cranial marrow cavity was visualized in transgenic mice expressing a targeted enhanced yellow fluorescent protein chimera fused to CD41, an integrin that is expressed exclusively in megakaryocytes and platelets. The investigators found that most megakaryocytes were perivascular and sessile. Treatment with thrombopoietin, however, increased the number of mature megakaryocytes demonstrating irregular shapes and exhibiting fragmented protrusions. Imaging also showed cellular processes extending into microvessels and releasing heterogeneous fragments resembling immature proplatelets into the bone marrow microvasculature. In addition, megakaryocytes extended plump perivascular pseudopodia into the microvessels. Shedding was accomplished primarily by large megakaryocytes with branched, irregular shapes. Quantitation of the frequency of shedding events indicated that this mechanism of platelet production accounted for the majority of circulating platelets.
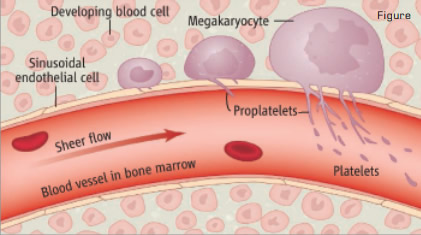
Junt, et al. Observed How Mature Megakaryocytes in Mouse Bone Marrow Routinely Extend Protrusions (Proplatelets) into Blood Vessels. Sheer stress from blood flow fragments these protrusions, generating platelets. Legend and figure from Geddis & Kaushansky, Science 317:1689 (2007). Illustration: Preston Huey. Reprinted with permission from AAAS.
Junt, et al. Observed How Mature Megakaryocytes in Mouse Bone Marrow Routinely Extend Protrusions (Proplatelets) into Blood Vessels. Sheer stress from blood flow fragments these protrusions, generating platelets. Legend and figure from Geddis & Kaushansky, Science 317:1689 (2007). Illustration: Preston Huey. Reprinted with permission from AAAS.
The observation that megakaryocytes extended into bone marrow microvessels to release immature proplatelets predicts that immature platelet forms will be found in the circulation. Indeed, evaluation of peripheral blood demonstrated multiple beaded proplatelets and barbell-shaped platelets. Previous studies have found that proplatelet counts are increased in prepulmonary arterial vessels while platelet counts are increased in post-pulmonary vessels. These observations indicate that platelet maturation continues following release of platelet fragments into the circulation.
In addition to demonstrating that megakaryocytes form proplatelets from extended pseudopodia in vivo, these studies suggest a role for shear force in thrombopoiesis. The observation that fragmentation of megakaryocytes occurred within blood vessels implicates shear force as a contributing factor. Furthermore, the investigators showed that megakaryocytes cultured with agitation demonstrated increased proplatelet formation compared with those cultured under static conditions.
In Brief
Visualization of megakaryocytes within bone marrow of living mice demonstrates that pseudopodia extend into the bone marrow vasculature and release fragments that subsequently mature into circulating platelets. These observations validate earlier studies that showed pseudopodia and proplatelet formation in cultured megakaryocytes. This work also implicates shear force as a contributing factor in megakaryocyte fragmentation and proplatelet maturation. The ability to visualize thrombopoiesis in vivo will provide for a more comprehensive analysis of thrombocytopenic states than has previous been possible. The effects of inflammatory conditions, infections, and congenital disorders on megakaryocyte maturation, proplatelet development, and shedding can now be evaluated directly and in live mammals. The in vivo model may also be useful in the characterization of thrombopoiesis-stimulating agents. The ability to visualize the events leading to platelet production in vivo is a landmark achievement and will, in a very real sense, change the way we look at thrombopoiesis.
Competing Interests
Dr. Flaumenhaft indicated no relevant conflicts of interest.