Natural killer cells are large granular lymphocytes that comprise approximately 10 percent of the peripheral blood mononuclear cell compartment. Despite sharing a close developmental relationship and similar cytolytic mechanisms with T cells, they are considered a part of the innate immune system. The designation "innate" is primarily one of exclusion; NK cells do not express the T-cell receptor or surface immunoglobulin, nor do they undergo V(D)J recombination, and consequently they are not part of the "adaptive" immune system. This and the observation that NK-like cells exist in more primitive animals while T and B cells can only be observed in vertebrates has led to the perception that NK cells are evolutionary forerunners of T and B cells.1,2 However, recent advances in our knowledge of NK-cell development, cellular interactions, and receptor biology have uncovered an entirely unexpected level of complexity in the biology of NK cells, which has important implications for the future of cancer immunotherapy.
Two immunophenotypically distinct subsets of NK cell can be found in human peripheral blood based on expression of CD56, a neural cell adhesion molecule (NCAM) and ligand for fibroblast growth factor receptor 1 (FGFR1): CD56bright and CD56dim NK.3 ,4 CD56dim cells produce more cytotoxic granules and are more effective in antibody-dependent cellular cytotoxicity and natural (antibody-independent) cytotoxicity than CD56bright NK cells.5 In contrast, CD56bright cells that are stimulated by monocyte-derived cytokines produce more interferon gamma, GM-CSF, IL-10, and IL-13 consistent with a role in regulating the immune response to infectious insult.6
Development
Like all hematopoietic cells, NK cells arise from pluripotent stem cells in the bone marrow. Early progenitor cells traffic to secondary lymphoid tissues where they pass through discrete developmental stages to the CD56bright NK-cell stage.7 A recent study has provided evidence for what may be the terminal event in NK-cell development.4 CD56bright NK cells were sorted and cultured on synovial or dermal fibroblast layers that express FGFR1. After several days in culture, the NK cells became CD56dim, had lost the ability to produce IFN-γ in response to cytokine stimulation, and had significantly increased capacity to participate in natural cytotoxicity. This result was independent of NK-cell proliferation but was dependent upon direct contact between the NK cells and the fibroblast layers. It was also inhibited by antibody blockade of the CD56-FGFR1 interaction. Although these findings have yet to be confirmed, a model has emerged wherein relatively immature CD56bright NK cells reside primarily in secondary lymphoid tissues and regulate local immune responses through cytokine production. These cells then migrate to the periphery where they differentiate into CD56dim NK and acquire the capacity for natural cytotoxicity.
Receptor Biology
The elucidation of the mechanism of NK-cell recognition of target cells dates back to their identification as lymphocytes that could kill "naturally" or without the need for prior antigenic exposure.8 In 1986, Karre provided a mechanism for these findings with his proposal of the "missing self hypothesis": NK cells are activated by target cells that downregulate expression of MHC class I,9 an event that often is associated with viral infection or malignant transformation. This hypothesis implies that NK cells express receptors for MHC class I that inhibit activation. Several groups identified expression of such receptors by both mouse and human NK cells.10-13 In humans, killer-cell Ig-like receptors (KIRs) are the primary inhibitory receptors for MHC class I. It appears that KIRs play an active role in the acquisition of cytolytic capacity during NK-cell development.14 In addition to inhibitory KIRs, there are a number of activating forms of KIR as well as activating and inhibitory receptors of the C-type lectin family including NKG2D and CD94/NKG2A.15 The integrated sum of activating and inhibitory inputs seems to determine the final outcome of the interaction between NK and target cell.15
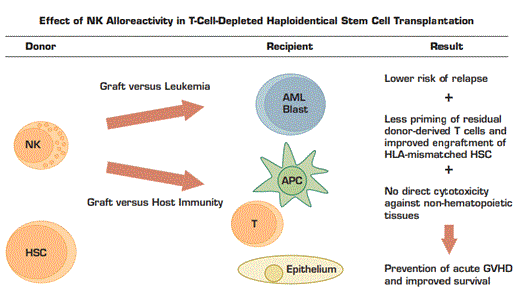
Alloreactive Donor-Derived NK Cells Preferentially Target AML Blasts, T Cells, and Antigen Presenting Cells (APC) Remaining in the Recipient After Conditioning for Transplantation. Recipients of alloreactive NK demonstrate lower rates of acute GVHD, graft rejection, leukemic relapse, and death after transplantation compared with recipients of non-alloreactive NK.
Alloreactive Donor-Derived NK Cells Preferentially Target AML Blasts, T Cells, and Antigen Presenting Cells (APC) Remaining in the Recipient After Conditioning for Transplantation. Recipients of alloreactive NK demonstrate lower rates of acute GVHD, graft rejection, leukemic relapse, and death after transplantation compared with recipients of non-alloreactive NK.
Clinical Application
More than thirty years of investigation into the biology of NK-cell development and signaling have led to important advances in clinical research in the treatment of leukemia. Based on pioneering work identifying the specificity of KIR for different HLA alleles,10,16 Velardi and others at the University of Perugia, Italy, showed that NK cells could efficiently lyse allogeneic leukemic blasts in vitro if KIR-HLA incompatibility were present.17 They have subsequently asked whether an NK-mediated graft-versus-leukemia (GVL) effect could be observed in vivo in recipients of haploidentical (multiply mismatched) allogeneic stem cell transplantation.18 Patients with AML in any complete remission or in chemo-resistant relapse were transplanted with highly T-cell-depleted and CD34+ stem-cell-enriched haploidentical allografts. Recipients with NK graft-versus-host alloreactivity as predicted by HLA-typing had significantly longer relapse-free survival and event-free survival than recipients of non-alloreactive NK. Strikingly, graft-versus-host NK alloreactivity did not increase the incidence of acute graft-versus-host disease (GVHD). Donor NK cells ignore non-hematopoietic tissues and preferentially eliminate recipient antigen-presenting cells, which are known to be the primary source of antigenic stimulation for donor-derived T cells.19-21 In addition to improving the relapse rate and preventing acute GVHD, alloreactive NK cells may also improve engraftment of HLA-incompatible hematopoietic stem cells (HSCs) by eliminating recipient T cells with the potential for graft rejection.19 Figure 1 summarizes these effects. The impressive results of the Perugia group have been attempted by others using various modifications to the protocol with mixed results, but more recent studies have used alternative T-cell depletion and stem-cell-enrichment strategies with increasing success.22
Ongoing translational and clinical research aims to bring the benefits of NK-cell anti-leukemic activity to a wider population of patients than only those who are candidates for allogeneic stem cell transplantation. By modulating the activity of KIRs or other families of NK cell receptors, it may be possible to induce a controlled level of NK autoimmunity that could reproduce the GVL effect without the associated risk of transplantation. In addition, our expanding knowledge of the immunologic and genetic control of NK developmental pathways may permit further manipulation of these cells through genetic, small molecule, or antibody-based approaches.
Conclusion
Once known primarily for what it could not do, the NK cell is now recognized as a unique and important part of the immune system with roles in infectious disease and tumor surveillance. Studies focused on the mechanisms of NK-target recognition have led to encouraging findings at the clinical level. As the basic and translational studies of today tell us more, our knowledge of the role NK cells in cancer immunotherapy will continue to unfold.