Bullinger L, Rücker FG, Kurz S, et al. Gene-expression profiling identifies distinct subclasses of core binding factor acute myeloid leukemia. Blood. 2007;110:1291-300.
As we move beyond a morphologic classification system of acute myeloid leukemia (AML) and into the era of genetic classifications for subgroups of AML, two of the major subgroups that have thus far emerged are those AML patients with disruptions of the core binding factor (CBF) complex and those patients with disruptions of the FLT3 gene.
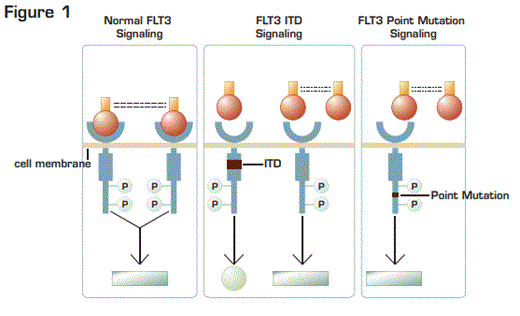
The FMS-like tyrosine kinase 3 (FLT3) gene is a membrane-bound receptor tyrosine kinase. Many hematopoietic cells produce FLT3 ligand, which promotes dimerization and activation of the receptor tyrosine kinase, FLT3. Similar to many cytokine-signaling pathways, upon activation FLT3 exerts positive effects on a multitude of downstream pathways including RAS and phosphatidylinositol 3-kinase (PI 3-K). There are two major classes of disruptions of the FLT3 gene in AML. Internal tandem duplications (ITDs) in the juxtamembrane domain involve head-to-tail duplication of 3-400 base pairs in exons 14 or 15 (Figure 1). FLT3 ITDs occur in 15 to 35 percent of patients with AML. The other major class is missense point mutations in exon 20 in the intracellular domain at D835. These missense point mutations occur in 5 to 10 percent of patients with AML.1 ,2
The CBF complex is a transcription factor complex critical for regulation of hematopoiesis and normal myeloid development. Disruptions of the CBF complex, t(8;21)(q22;q22) or inv(16)(p13q22)/ t(16,16)(p13;q22), constitute AML subgroups with favorable prognosis. The problem exists that in both the FLT3 and CBF groups of patients with AML considerable clinical heterogeneity exists that implies there must be additional biologic or genetic heterogeneity. Three manuscripts, all in the August 15, 2007, issue of Blood, shed new light on these subgroups and also highlight some possible interactions between genetic lesions.
Mead and colleagues screened 1,107 young adult patients with AML for FLT3 ITDs and for FLT3 point mutations (utilizing dHPLC analysis). They found a 23 percent incidence for FLT3 ITDs alone, 9 percent incidence for FLT3 point mutations alone, and, interestingly, 2 percent of patients that had both kinds of FLT3 aberrancies. There was a highly significant difference between patients with FLT3 ITD and patients with an FLT3 point mutation, both in terms of cumulative incidence of relapse and overall survival, with FLT3 ITD patients having a poorer prognosis in all analyses.
Bullinger and colleagues utilized gene-expression profiling to examine 93 AML patients with disruptions in the CBF complex, 55 with inv(16), and 38 with t(8;21). By unsupervised hierarchical clustering, they identified two subgroups of CBF AML patients based on distinct patterns of gene expression. The "unfavorable" subgroup was associated with elevated white blood cell counts and FLT3 ITDs. The gene-expression signatures associated with this "unfavorable" group included proliferative-type genes such as JUN, FOS, and others in the MAPK pathway as well as high-level expression of genes involved in the response to DNA damage and in DNA repair. Conversely, the "favorable" subgroup of the CBF AML patient samples was characterized by prominent gene-expression features of antiapoptotic pathways.
Finally, Dicker and colleagues investigated AML1/RUNX1 gene mutations as a part of disruption of the CBF complex. In different patient cohorts they noted a recurring theme of RUNX1 mutations being associated with trisomy 13 independent of the FAB subgroup. Since the FLT3 gene is localized on chromosome 13, they hypothesized that RUNX1 mutations might cooperate with trisomy 13 by increasing FLT3 transcript levels. These results pointed to a potential third type of involvement in AML by FLT3 gene abnormalities, namely, in the absence of ITDs or point mutations, FLT3 gene overexpression could be a third route for FLT3 activation, which could potentially cooperate in leukemic transformation together with RUNX1 (CBF) mutations.
In Brief
Overall, these three papers teach us several things. First, that the FLT3 tyrosine kinase can be involved in leukemogenesis by a number of different genetic mechanisms. Secondly, it teaches us that even if it’s the same gene being activated, the different mechanisms for activation can have significantly different phenotypic behaviors. And finally, that the two major subgroups of genetic disruptions in AML (CBF complex and FLT3) can demonstrate interaction in initiating and maintaining the leukemic clone.
References
Competing Interests
Dr. Emanuel indicated no relevant conflicts of interest.