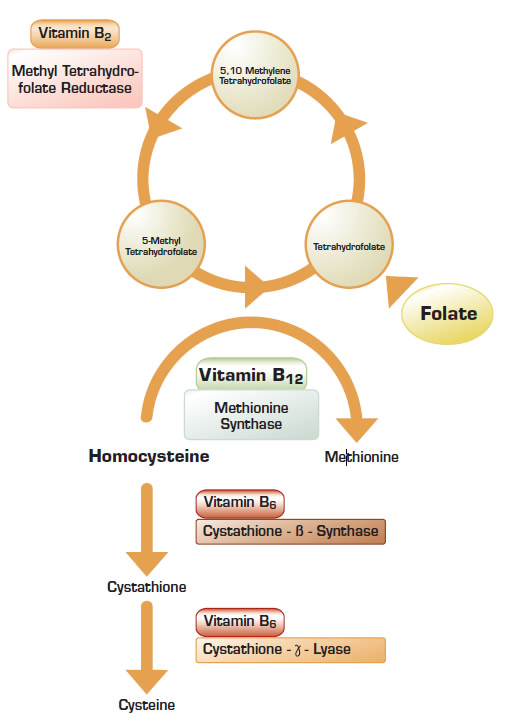
The Heart Outcomes Prevention Evaluation 2 (HOPE-2) trial was designed to evaluate the effect of lowering homocysteine on the development of arterial vascular disease. In this five-year study, a daily combination of folate, vitamin B6, and vitamin B12 was administered to 2,758 participants, while another 2,764 participants took matching placebos. In conjunction with this trial, separate analysis was done to determine whether decreasing plasma homocysteine levels reduced the occurrence of symptomatic venous thromboembolic disease.
Of the 5,522 participants, 72 percent were from the United States and Canada where food is fortified with folate. Consequently, the mean plasma homocysteine level at entry into the trial was only 11.5 mmol/l. At the end of the trial, the mean homocysteine level decreased by 2.2 mmol/l in the treatment group and increased by 0.8 mmol/l in the control group. It is notable that in the treatment group, the mean homocysteine level decreased by 1.9 mmol/l in participants from regions with folate food fortification and decreased by 4.8 mmol/l in participants from regions that do not fortify food with folate. This suggests that patients in regions without folate-rich diets derive the most benefit from vitamin supplementation. Despite changes in plasma homocysteine levels, the treatment and the control groups had an identical number (44) of symptomatic venous thromboembolisms. Restricting the analysis to the 821 participants who had plasma homocysteine levels in the highest quartile (>13.8 mmol/l) still did not show a benefit for folate therapy.
In Brief
Homocystinuria is a rare inborn error of metabolism associated with extremely high plasma homocysteine levels, premature cardiovascular disease, and developmental abnormalities of the ocular, skeletal, and nervous systems. Less significant elevation of plasma homocysteine is common and is not associated with developmental abnormalities. Analysis of patients and matched control subjects participating in the Leiden Thrombophilia Study suggested that plasma homocysteine levels above the 95th percentile in the control group (>18.5 mmol/l) is a risk factor for venous thrombosis1 . It is notable that there was a very high risk for thromboembolic events in participants with homocysteine levels greater than 22 mmol/l. Since modest elevations of plasma homocysteine to less than 18.0 mmol/l were not associated with any increased risk of thrombosis, data from the Leiden study suggested that there might be a threshold level when homocysteine may induce venous thrombosis.
The results of the HOPE-2 trial failed to demonstrate a benefit for homocysteine lowering therapy in the analyzed cohorts. Does this mean that homocysteine is never a risk factor for thromboembolic disease, and patients with hyperhomocysteine will not benefit from folate supplementation? The patients enrolled in the HOPE-2 study, as well as the patients enrolled in the recently published VITRO study2 , were selected for their overall cardiovascular risk, not their baseline plasma homocysteine levels. Consequently, there were very few analyzed participants with plasma homocysteine levels above the critical threshold as proposed by the Leiden Thrombophilia Study group. Therefore, it is still unclear whether the risk of thromboembolic events in patients with very high plasma homocysteine levels would be reduced by a combination of folate, vitamin B6, and vitamin B12.
What does all this mean for mothers (and physicians) who recommend eating lots of veggies? First, it emphasizes that food in the United States and Canada that is fortified with folate has made significant hyperhomocysteinemia a rare occurrence in these countries. Second, patients with mild hyperhomocysteinemia do not benefit from therapy designed to reduce plasma homocysteine. Third, it is still unknown whether the exceptional patient with a plasma homocysteine level greater than 22 mmol/l would still benefit from folate therapy, and this demands further study. However, given the available data and the minimal toxicity of therapy, it appears that treatment for these select few is for now still a worthwhile endeavor.
References:
References
Competing Interests
Dr. Abrams indicated no relevant conflicts of interest.