There is rapidly emerging evidence of dual and often unexpected functions of many human proteins. The transferrin receptor 1 (TfR1) is the essential host protein that efficiently delivers iron to erythroid progenitors and other rapidly proliferating cells. In this paper, Radoshitzky and colleagues in Choe’s laboratory uncover another such dual function protein; the piggy-backing of an infectious agent onto this essential human protein. Viral hemorrhagic fevers are rare but devastating disorders that can be caused by at least five different arenaviruses. Four of these arenaviruses are classified as New World viruses, since the rodent hosts are located in the Western hemisphere. While these viruses are asymptomatic in the rodent host, when humans are infected the mortality rate can reach 30 percent. These investigators have identified the TfR1 as the protein responsible for binding and internalization of these viruses. The viral coat protein GP1 mediates the binding to the host cell surface. To identify which cell-surface protein was functioning as the viral receptor, the authors constructed a fusion protein consisting of the viral GP1 fused with the Fc portion of IgG1. This fusion protein was used to immunoprecipitate potential receptor proteins from metabolically labeled endothelial cells from African green monkeys, which are highly susceptible to these viruses. The major band immunoprecipitated by the fusion protein was characterized using mass spectroscopy and identified as TfR1. Chinese hamster ovary cells are resistant to New World arenavirus infections, but inducing expression of human TfR1 in these cells rendered them permissive for infection with the various New World viruses, confirming the essential role of TfR1. On the other hand, expression of the closely related transferrin receptor 2 did not allow viral binding and entry. Modulation of the TfR1 levels by treating the cells with iron or iron chelators showed that infectivity with the arenavirus was directly proportional to the quantity of TfR1 on the cell surface.
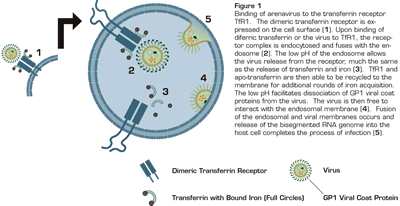
Binding of Arenavirus to the Transferrin Receptor TfR1. The dimeric transferrin receptor is expressed on the cell surface (1). Upon binding of diferric transferrin or the virus to TfR1, the receptor complex is endocytosed and fuses with the endosome (2). The low pH of the endosome allows the virus release from the receptor, much the same as the release of transferrin and iron (3). TfR1 and apo-transferrin are then able to be recycled to the membrane for additional rounds of iron acquisition. the low pH facilitates dissociation of GP1 viral coat proteins from the virus. The virus is then free to interact with the endosomal membrane (4). Fusion of the endosomal and viral membranes occurs and release of the bisegmented RNA genome into the host cell completes the process of infection (5).
Binding of Arenavirus to the Transferrin Receptor TfR1. The dimeric transferrin receptor is expressed on the cell surface (1). Upon binding of diferric transferrin or the virus to TfR1, the receptor complex is endocytosed and fuses with the endosome (2). The low pH of the endosome allows the virus release from the receptor, much the same as the release of transferrin and iron (3). TfR1 and apo-transferrin are then able to be recycled to the membrane for additional rounds of iron acquisition. the low pH facilitates dissociation of GP1 viral coat proteins from the virus. The virus is then free to interact with the endosomal membrane (4). Fusion of the endosomal and viral membranes occurs and release of the bisegmented RNA genome into the host cell completes the process of infection (5).
This paper also clarifies the previously reported low pH requirement for fusion of these arenoviruses with host cell membrane1 . The transferrin receptor/transferring-bound iron complex is internalized in endosomes where the low pH facilitates release of iron and transferrin from TfR1 and the recycling of the receptor to the cell surface. The same mechanism allows release of virus, modification of the viral coat, and fusion of the endosomal and viral membranes (Figure 1). The TfR1 is highly expressed on endothelial cells and at high levels on dividing macrophages and lymphocytes (which increase during infection), further facilitating infectivity and replication of the virus. Thus, arenaviruses hijacked a critical process of nutrient acquisition to provide a route of entry into the cell.
In Brief
This manuscript expands our knowledge of disease states that are linked to iron metabolism and infection. These links include the association between the detrimental use of iron for an undiagnosed anemia that may worsen the outcome of bacterial and mycobacterial infections, the salutary effect of iron chelators on cancer progression, and the lipocalin-iron pathway that regulates apoptosis of certain cancers2 . The use of TfR1 as a viral entry receptor has also been demonstrated in other species (i.e., mouse mammary tumor virus has been shown to use TfR1 for entry, as have canine and feline parvoviruses). With the high degree of homology between the transferrin receptors, it is remarkable how species-specific the viral coat proteins are. As pointed out by the authors, the effect of iron deficiency is not clear on the infectious outcome of these viruses. While iron deficiency increases TfR1 expression and thus infectivity, the soluble TfR1 that also increases may, in fact, act as a decoy and protect the cells from viral entry. However, iron therapy during an acute infection may be beneficial by decreasing both the level of cell surface and the circulating TfR1. A novel therapeutic modality that remains to be tested — the administration of anti-TfR1 humanized antibodies (that are already being evaluated as the anti-cancer agents3 ) would be expected to be beneficial in patients infected with New World hemorrhagic fever.
References
Competing Interests
Drs. Phillips and Prchal indicated no relevant conflicts of interest.