The first studies linking a variant form of Creutzfeldt-Jakob disease (vCJD) to transmission of bovine spongiform encephalopathy (BSE) were published a decade ago. At that time, measures were taken to prevent exposure to contaminated cattle-derived food products, and programs were implemented to both minimize the theoretical risk of prion transmission by transfusion and to monitor recipients of blood products from donors who were subsequently diagnosed with vCJD. This paper describes a third case of autopsy-confirmed prion infection, and the second case of clinical vCJD, identified within a cohort of 66 at-risk transfusion recipients followed by the U.K. Transfusion Medicine Epidemiological Review (TMER) study. The patient was a 31-year-old man with ulcerative colitis who developed neurological symptoms six years after receiving non-leukodepleted red cells from an individual who was diagnosed with vCJD at 20 months after donation. The patient suffered progressive cognitive impairment, leg dysesthesias, ataxia, and dysarthria. An MRI scan eventually revealed changes in the posteromedial thalamus typical of the pulvinar sign associated with vCJD. The patient died in hospice after a 32-month course, having failed a therapeutic trial of quinacrine. Pre-mortem sequencing of PRNP, the gene encoding the prion precursor protein (PrPc), ruled out mutations associated with inherited prion disease. He was homozygous for methionine (M/M) at codon 129, a polymorphism found in all vCJD cases and implicated in disease predisposition and pathogenesis. Post-mortem analyses confirmed the presence of misfolded prion protein (PrPSc) in tissue homogenates of tonsil and brain. Abnormal PrP was identified in immunohistochemical sections of the tonsil and within areas of gliosis and plaques in brain cortical sections.
In Brief
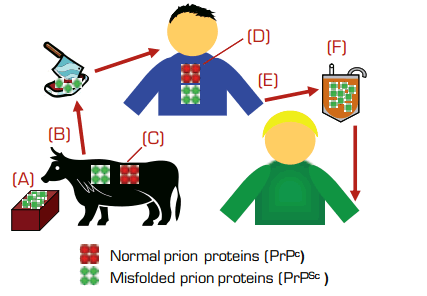
This case, along with the post-mortem detection of infection in two other recipients from the TMER cohort (one of whom died with neurodegenerative symptoms consistent with vCJD), strongly reinforces the concern that pathogenic prion proteins may be transmitted through blood transfusions. The donors in these cases developed their clinical symptoms of vCJD at 18, 20, and 40 months following donation. The two recipients with vCJD were homozygous for M/M at codon 129 (the asymptomatic, infected recipient was heterozygous for methionine and valine), and they developed symptoms at six and 6.5 years after transfusion. Notably, of the 32 individuals in the original TMER cohort who survived beyond five years after transfusion, two developed clinical vCJD. Thus, pre-clinical infection appears to be sufficient for blood transmission; the “incubation period” for transfusion-associated prion disease is likely shorter than that for food-borne vCJD (which is estimated to be decades); infectivity rates may be high, at least under certain donor and/or recipient conditions; and recipient genotype at codon 129 may be an important disease-modulating factor. Given the lack of a blood assay for infection, uncertainty about prion tropism and host susceptibility, and incomplete knowledge of disease pathogenesis, a number of preventative measures have been in effect, and additional interventions are in various stages of development. These include (see Figure): (A) elimination of potentially contaminated cattle feed; (B) removal of older and diseased animals from the food chain; (C) generation of cloned cattle that lack the normal PrPc protein1 , which is required to propagate misfolded PrPSc prions; (D) treatment of infected individuals with agents that reduce PrPc production, such as lentiviral vector-mediated transfer of small interfering RNAs2; (E) screening and deferral policies to prevent horizontal transmission from potentially infected blood donors; and (F) removal of infectious prions from blood-derived products by leukodepletion, filtration, resin adsorption3 , and/or biophysical purification. Although prion diseases are a growing concern worldwide, the urgency for progress is greatest for the U.K. and France, which account for 85 percent and 10 percent, respectively, of all the vCJD cases reported to date. The blood donor populations in those countries are estimated to include the highest numbers of individuals with asymptomatic, undetected prion infections.
As The Hematologist was going to press, a fourth case of transfusion-associated prion infection, and a third case of probable vCJD, was reported among a surviving member of the TMER recipient cohort (see http://www.hpa.org.uk/infections/topics_az/cjd/vCJDBloodDonors.htm#nfn).
References
Competing Interests
Dr. Linenberger indicated no relevant conflicts of interest.