This is a phase I/II trial that tests the hypothesis that inducing increased platelet production would be beneficial in ITP. AMG 531 binds and activates the thrombopoietin (TPO) receptor, but bears no structural similarity to TPO. Therefore, it is a TPO-mimetic and, in theory, should increase platelet production in man. In the first phase, 24 patients were exposed to two injections of escalating doses of AMG 531. Doses greater than 3 micrograms/kg induced an increase to some extent in platelet counts in all subjects. All doses up to 10 micrograms/kg were well tolerated. In phase II, 16 long-term ITP patients were enrolled in a double-blind, placebo-controlled trial of AMG 531. Patients were treated with six weekly subcutaneous injections of 1 or 3 micrograms/kg of the study drug. AMG 531 increased the platelet count to greater than 50,000/µl in twelve patients (a 75 percent response rate). Notably, two patients who received 3 micrograms/kg of AMG 531 had platelet counts that increased above 500,000/µl. Interestingly, there appeared to be no relationship between baseline TPO levels and platelet responses. This implies that TPO-mimetics are able to overdrive platelet production even in patients with appropriate (i.e., normal) TPO levels. Reassuringly, the overall toxicity was low.
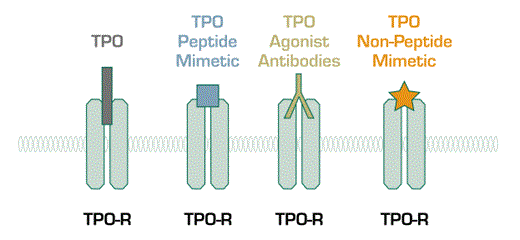
Thrombo-Mimetics. Shown are three classes of pharmaceuticals that share little homology to thrombopoietin (TPO), yet sti l activate the TPO-receptor (TPO-R).
Thrombo-Mimetics. Shown are three classes of pharmaceuticals that share little homology to thrombopoietin (TPO), yet sti l activate the TPO-receptor (TPO-R).
In Brief
Although the majority of ITP patients have a compensatory increase in megakaryopoiesis, plasma from some ITP patients can inhibit platelet production ex vivo. These data suggest that, in addition to increased platelet destruction, impaired megakaryopoiesis is another reason for the thrombocytopenia in this disease. The fact that TPO levels in the plasma of ITP patients are not as high as TPO levels in patients with other types of thrombocytopenia suggests that there might be room to overdrive platelet production in ITP patients by administration of exogenous TPO. Several years ago, a small trial demonstrated that platelet counts could indeed be significantly increased in some ITP patients exposed to a truncated form of recombinant thrombopoietin (PEG-MGDF)1 . Unfortunately, PEG-MGDF-induced auto-antibodies against endogenous thrombopoietin, which ultimately necessitated the discontinuation of further product development.
Several investigators proposed the hypothesis that molecules that bear no structural resemblance to TPO, but still bind and activate the TPO receptor (so called TPO mimetics), might be useful for the treatment of ITP without the risk of inducing anti-TPO antibodies. Since that time, a few groups have been able to identify peptides that bind the TPO receptor with high affinity. AMG 531 is the most developed pharmaceutical in the TPO mimetic category. It is composed of several copies of a TPO receptor-binding peptide spliced into a recombinant antibody. This peptide mimetic competes with thrombopoietin for binding to the TPO receptor and activates the receptor in an identical fashion to endogenous thrombopoietin. As shown in this paper, AMG 531 produces a dose-dependent increase in platelet counts in ITP patients.
A similar approach has been used to identify other small molecules that bear little structural similarity to TPO, yet are still capable of binding and activating the TPO receptor. Screening small-molecule libraries for compounds that have TPO-like activity identified these so-called TPO nonpeptide mimetics. The most developmentally advanced of this category is Eltrombopag. Like subcutaneously administered AMG 531, oral Eltrombopag also produces a dose-dependent increase in the platelet counts of both healthy volunteers and patients with ITP. Although less far along in clinical development, orally administered AKR-501 also functions by a similar mechanism. A final class of drugs that stimulate the TPO receptor are agonist antibodies, although these as well are not far along in clinical development.
Although the data on TPO mimetics are currently tantalizing, the long-term effects of these compounds remain to be established. Of concern is the development of reticulin bone marrow fibrosis in one patient who took AMG 531 for an extended period of time. Thus far, this specific toxicity has been reversible after discontinuation of the medication. Also notable is that TPO can increase the sensitivity of platelets to standard platelet agonists in vitro. Therefore, whether these agents will lead to thrombotic complications over time remains to be seen. But, for now, the news is good. Trials of TPO-mimetics appear to be heralding a new era of non-immunosuppresive therapy for ITP.
References
Competing Interests
Dr. Abrams is an ad hoc consultant to GlaxoSmithKline.